当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bio-inspired protonic memristor devices based on metal complexes with proton-coupled electron transfer
Faraday Discussions ( IF 3.3 ) Pub Date : 2018-07-02 , DOI: 10.1039/c8fd00098k Yusuke Hiruma 1, 2, 3, 4, 5 , Kai Yoshikawa 1, 2, 3, 4, 5 , Masa-aki Haga 1, 2, 3, 4, 5
Faraday Discussions ( IF 3.3 ) Pub Date : 2018-07-02 , DOI: 10.1039/c8fd00098k Yusuke Hiruma 1, 2, 3, 4, 5 , Kai Yoshikawa 1, 2, 3, 4, 5 , Masa-aki Haga 1, 2, 3, 4, 5
Affiliation
|
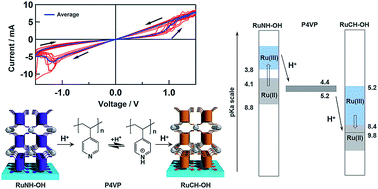
A new type of memristor inspired by bio-membranes is presented, based on the proton movement resulting from proton-coupled electron transfer (PCET) processes in dinuclear Ru complexes, whereby a two-terminal device based on said Ru complexes and a proton-conducting polymer was constructed as a proof-of-concept. Two ITO electrodes were modified separately with dinuclear Ru complexes that bear tetraphosphonic acid linkers at both ends and a 2,6,2′,6′-tetrakis(benzimidazol-2-yl)-4,4′-bipyridine (RuNH-OH) or 1,3,1′,3′-tetrakis(benzimidazol-2-yl)-5,5′-biphenyl (RuCH-OH) bridging ligand, and both ITO electrodes exhibit PCET processes with different Ru(II/III) redox potentials and pKa values. Poly(4-vinylpyridine) (P4VP; pKa = 4–5), a proton-conducting polymer, was sandwiched between the two modified ITO electrodes to construct a two-terminal device of the type ITO|(RuNH-OH)3|P4VP|(RuCH-OH)3|ITO. Initially, the oxidation state of the metal centers in RuNH-OH and RuCH-OH is Ru(II) and Ru(III), respectively. Upon applying a bias voltage between the two ITO electrodes, the high and low current states switch at approximately ±1.10 V due to Ru(II/III) redox reactions. At the RuNH-OH|P4VP and RuCH-OH|P4VP interfaces, a proton is released from Ru(II)NH-OH and subsequently captured by Ru(III)CH-OH through the hydrogen-bonding interaction with the P4VP polymer, which is driven by the changes in the pKa values of the Ru complexes from 4.1–8.8 [Ru(II)NH-OH] to <3.8 [Ru(III)NH-OH] and from <8.4 [Ru(II)CH-OH] to 5.2–9.8 [Ru(III)CH-OH] under these conditions. The redox reactions on the modified Ru films create a large proton gradient between the two electrodes, enhancing the proton conductivity through the P4VP layer (pKa = 4–5). When the applied bias potential was inverted, the pKa gradient returned to the original state and the current decreased. Such a proton-conductivity enhancement is relevant to the transport of protons by proton gradients in bio-membranes. Therefore, the present protonic coordination-network films containing metal complexes that exhibit PCET should open new avenues for the design of a new type of memristor devices mimicking the function of synapses.
中文翻译:

基于金属的质子耦合电子转移的生物启发质子忆阻器
提出了一种新型的受生物膜启发的忆阻器,它基于在双核Ru络合物中的质子耦合电子转移(PCET)过程产生的质子运动,其中基于Ru络合物和质子传导的两末端装置聚合物被构造为概念证明。两个ITO电极分别用双核Ru配合物修饰,该配合物在两端带有四膦酸连接基,并带有2,6,2',6'-四(苯并咪唑-2-基)-4,4'-联吡啶(RuNH-OH)或1,3,1',3'-四(苯并咪唑-2-基)-5,5'-联苯(RuCH-OH)桥联配体,并且两个ITO电极均具有不同的Ru(II / III)氧化还原的PCET工艺势和p K a价值观。聚(4-乙烯基吡啶)(P4VP; p K a = 4-5),质子传导聚合物,被夹在两个修饰的ITO电极之间,以构造ITO |(RuNH-OH)3型的双端子器件| P4VP |(RuCH-OH)3 | ITO。最初,RuNH-OH和RuCH-OH中金属中心的氧化态分别为Ru(II)和Ru(III)。在两个ITO电极之间施加偏置电压后,由于Ru(II / III)氧化还原反应,高电流状态和低电流状态在大约±1.10 V处切换。在RuNH-OH | P4VP和RuCH-OH | P4VP界面上,质子从Ru(II)NH-OH中释放出来,随后通过与P4VP聚合物的氢键相互作用被Ru(III)CH-OH捕获,这是由p的变化驱动的Ru络合物的K a值从4.1–8.8 [ Ru(II)NH-OH ]到<3.8 [ Ru(III)NH-OH ]和从<8.4 [ Ru(II)CH-OH ]到5.2–9.8 [钌(III)CH-OH在这些条件下。改性Ru膜上的氧化还原反应在两个电极之间产生大的质子梯度,从而增强了通过P4VP层的质子传导性(p K a = 4-5)。当施加的偏置电势反转时,p K a梯度返回到原始状态,电流减小。这种质子电导率的提高与生物膜中质子梯度对质子的运输有关。因此,目前的含有显示PCET的金属配合物的质子配位网络薄膜应该为设计新型的模仿突触功能的忆阻器装置开辟新的途径。
更新日期:2019-02-19
中文翻译:

基于金属的质子耦合电子转移的生物启发质子忆阻器
提出了一种新型的受生物膜启发的忆阻器,它基于在双核Ru络合物中的质子耦合电子转移(PCET)过程产生的质子运动,其中基于Ru络合物和质子传导的两末端装置聚合物被构造为概念证明。两个ITO电极分别用双核Ru配合物修饰,该配合物在两端带有四膦酸连接基,并带有2,6,2',6'-四(苯并咪唑-2-基)-4,4'-联吡啶(RuNH-OH)或1,3,1',3'-四(苯并咪唑-2-基)-5,5'-联苯(RuCH-OH)桥联配体,并且两个ITO电极均具有不同的Ru(II / III)氧化还原的PCET工艺势和p K a价值观。聚(4-乙烯基吡啶)(P4VP; p K a = 4-5),质子传导聚合物,被夹在两个修饰的ITO电极之间,以构造ITO |(RuNH-OH)3型的双端子器件| P4VP |(RuCH-OH)3 | ITO。最初,RuNH-OH和RuCH-OH中金属中心的氧化态分别为Ru(II)和Ru(III)。在两个ITO电极之间施加偏置电压后,由于Ru(II / III)氧化还原反应,高电流状态和低电流状态在大约±1.10 V处切换。在RuNH-OH | P4VP和RuCH-OH | P4VP界面上,质子从Ru(II)NH-OH中释放出来,随后通过与P4VP聚合物的氢键相互作用被Ru(III)CH-OH捕获,这是由p的变化驱动的Ru络合物的K a值从4.1–8.8 [ Ru(II)NH-OH ]到<3.8 [ Ru(III)NH-OH ]和从<8.4 [ Ru(II)CH-OH ]到5.2–9.8 [钌(III)CH-OH在这些条件下。改性Ru膜上的氧化还原反应在两个电极之间产生大的质子梯度,从而增强了通过P4VP层的质子传导性(p K a = 4-5)。当施加的偏置电势反转时,p K a梯度返回到原始状态,电流减小。这种质子电导率的提高与生物膜中质子梯度对质子的运输有关。因此,目前的含有显示PCET的金属配合物的质子配位网络薄膜应该为设计新型的模仿突触功能的忆阻器装置开辟新的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号