当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adjusting the Structure of 2′-Modified Nucleosides and Oligonucleotides via C4′-α-F or C4′-α-OMe Substitution: Synthesis and Conformational Analysis
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.joc.8b01329 Elise Malek-Adamian 1 , Mihai Burai Patrascu 1 , Sunit Kumar Jana 1 , Saúl Martínez-Montero 1 , Nicolas Moitessier 1 , Masad J. Damha 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.joc.8b01329 Elise Malek-Adamian 1 , Mihai Burai Patrascu 1 , Sunit Kumar Jana 1 , Saúl Martínez-Montero 1 , Nicolas Moitessier 1 , Masad J. Damha 1
Affiliation
|
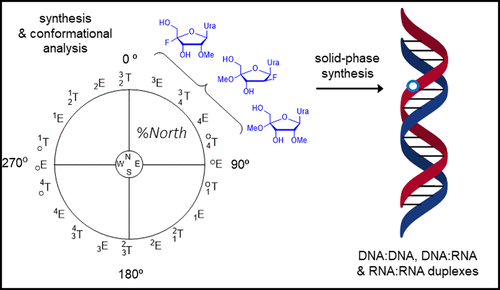
We report the first syntheses of three nucleoside analogues, namely, 2′,4′-diOMe-rU, 2′-OMe,4′-F-rU, and 2′-F,4′-OMe-araU, via stereoselective introduction of fluorine or methoxy functionalities at the C4′-α-position of a 4′,5′-olefinic intermediate. Conformational analyses of these nucleosides and comparison to other previously reported 2′,4′-disubstituted nucleoside analogues make it possible to evaluate the effect of fluorine and methoxy substitution on the sugar pucker, as assessed by NMR, X-ray diffraction, and computational methods. We found that C4′-α-F/OMe substituents reinforce the C3′-endo (north) conformation of 2′-OMe-rU. Furthermore, the predominant C2′-endo (south/east) conformation of 2′-F-araU switches to C3′-endo upon introduction of these substituents at C4′. The nucleoside analogues were incorporated into DNA and RNA oligonucleotides via standard phosphoramidite chemistry, and their effects on the thermal stability of homo- and heteroduplexes were assessed via UV thermal melting experiments. We found that 4′-substituents can modulate the binding affinity of the parent 2′-modified oligomers, inducing a mildly destabilizing or stabilizing effect depending on the duplex type. This study expands the spectrum of oligonucleotide modifications available for rational design of oligonucleotide therapeutics.
中文翻译:

通过C4'-α-F或C4'-α-OMe取代调节2'-修饰的核苷和寡核苷酸的结构:合成和构象分析
我们通过立体选择性介绍报告了三个核苷类似物,即2',4'-diOMe-rU,2'-OMe,4'-F-rU和2'-F,4'-OMe-araU的首次合成。氟在4',5'-烯烃中间体的C4'-α-位上的官能度。这些核苷的构象分析以及与其他先前报道的2',4'-双取代核苷类似物的比较,使得可以通过NMR,X射线衍射和计算方法评估氟和甲氧基取代对糖皱的影响。我们发现,C4'-α-F/ OMe取代基增强了2'-OMe-rU的C3' -endo(北方)构象。此外,主要的C2'-endo(南/东)在这些取代基在C4'处引入后,2'-F-araU的构象转换为C3'-endo。核苷类似物通过标准亚磷酰胺化学方法掺入DNA和RNA寡核苷酸中,并通过UV热熔实验评估它们对同双链和异双链热稳定性的影响。我们发现4'-取代基可以调节亲本2'-修饰的低聚物的结合亲和力,根据双链体类型诱导适度的去稳定化或稳定化作用。这项研究扩大了寡核苷酸修饰的范围,可用于合理设计寡核苷酸治疗药物。
更新日期:2018-07-02
中文翻译:

通过C4'-α-F或C4'-α-OMe取代调节2'-修饰的核苷和寡核苷酸的结构:合成和构象分析
我们通过立体选择性介绍报告了三个核苷类似物,即2',4'-diOMe-rU,2'-OMe,4'-F-rU和2'-F,4'-OMe-araU的首次合成。氟在4',5'-烯烃中间体的C4'-α-位上的官能度。这些核苷的构象分析以及与其他先前报道的2',4'-双取代核苷类似物的比较,使得可以通过NMR,X射线衍射和计算方法评估氟和甲氧基取代对糖皱的影响。我们发现,C4'-α-F/ OMe取代基增强了2'-OMe-rU的C3' -endo(北方)构象。此外,主要的C2'-endo(南/东)在这些取代基在C4'处引入后,2'-F-araU的构象转换为C3'-endo。核苷类似物通过标准亚磷酰胺化学方法掺入DNA和RNA寡核苷酸中,并通过UV热熔实验评估它们对同双链和异双链热稳定性的影响。我们发现4'-取代基可以调节亲本2'-修饰的低聚物的结合亲和力,根据双链体类型诱导适度的去稳定化或稳定化作用。这项研究扩大了寡核苷酸修饰的范围,可用于合理设计寡核苷酸治疗药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号