Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-07-02 , DOI: 10.1016/j.bmc.2018.07.001 Yiming Xu , Dewang Jing , Rui Chen , Haroon Ur Rashid , Jun Jiang , Xu Liu , Lisheng Wang , Peng Xie
|
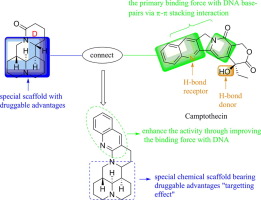
Based on our previous study and the binding mode of camptothecin with Topo I, a series of novel sophoridine imine derivatives containing conjugated planar structure were designed, synthesized and tested for their in vitro anticancer activity. The results showed that most of the derivatives displayed potent activity. In particular, compounds 10b exhibited excellent anti-proliferative activities with IC50 5.7 µM and 8.5 µM against HepG-2 and HeLa cell lines, respectively. Molecular docking studies revealed that the introduction of conjugated planar structure could form π-π stacking interaction with DNA, leading to the improvement of biological activity. Its mode of action was to inhibit the activity of DNA Topo I, followed by the G0/G1 phase arrest. This work provides a theoretical basis for structural optimizations and exploring anticancer pathways of this kind of compound and 10b could emerge as promising lead compounds for the development of novel Topo I inhibitors.
中文翻译:

以共轭平面结构为有效抗癌剂的新型槐定亚胺衍生物的设计,合成和评价
基于我们先前的研究和喜树碱与Topo I的结合模式,设计,合成并测试了一系列新型的具有共轭平面结构的槐定亚胺衍生物的体外抗癌活性。结果表明,大多数衍生物显示出有效的活性。尤其是,化合物10b对IC 50表现出优异的抗增殖活性分别针对HepG-2和HeLa细胞系的5.7 µM和8.5 µM。分子对接研究表明,引入共轭平面结构可与DNA形成π-π堆积相互作用,从而提高了生物活性。其作用方式是抑制DNA Topo I的活性,然后抑制G0 / G1相。这项工作为结构优化提供了理论基础,并探索了这种化合物的抗癌途径,而10b有望成为开发新型Topo I抑制剂的有前途的先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号