Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microphysiological flux balance platform unravels the dynamics of drug induced steatosis.
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-08-21 , DOI: 10.1039/c8lc00357b Avner Ehrlich 1 , Sabina Tsytkin-Kirschenzweig , Konstantinos Ioannidis , Muneef Ayyash , Anne Riu , Reine Note , Gladys Ouedraogo , Jan Vanfleteren , Merav Cohen , Yaakov Nahmias
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-08-21 , DOI: 10.1039/c8lc00357b Avner Ehrlich 1 , Sabina Tsytkin-Kirschenzweig , Konstantinos Ioannidis , Muneef Ayyash , Anne Riu , Reine Note , Gladys Ouedraogo , Jan Vanfleteren , Merav Cohen , Yaakov Nahmias
Affiliation
|
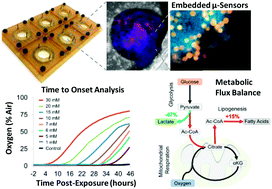
Drug development is currently hampered by the inability of animal experiments to accurately predict human response. While emerging organ on chip technology offers to reduce risk using microfluidic models of human tissues, the technology still mostly relies on end-point assays and biomarker measurements to assess tissue damage resulting in limited mechanistic information and difficulties to detect adverse effects occurring below the threshold of cellular damage. Here we present a sensor-integrated liver on chip array in which oxygen is monitored using two-frequency phase modulation of tissue-embedded microprobes, while glucose, lactate and temperature are measured in real time using microfluidic electrochemical sensors. Our microphysiological platform permits the calculation of dynamic changes in metabolic fluxes around central carbon metabolism, producing a unique metabolic fingerprint of the liver's response to stimuli. Using our platform, we studied the dynamics of human liver response to the epilepsy drug Valproate (Depakine™) and the antiretroviral medication Stavudine (Zerit™). Using E6/E7LOW hepatocytes, we show TC50 of 2.5 and 0.8 mM, respectively, coupled with a significant induction of steatosis in 2D and 3D cultures. Time to onset analysis showed slow progressive damage starting only 15-20 hours post-exposure. However, flux analysis showed a rapid disruption of metabolic homeostasis occurring below the threshold of cellular damage. While Valproate exposure led to a sustained 15% increase in lipogenesis followed by mitochondrial stress, Stavudine exposure showed only a transient increase in lipogenesis suggesting disruption of β-oxidation. Our data demonstrates the importance of tracking metabolic stress as a predictor of clinical outcome.
中文翻译:

微生理通量平衡平台揭示了药物性脂肪变性的动力学。
目前,动物实验无法准确预测人类的反应,阻碍了药物的研发。尽管新兴的器官上芯片技术使用人体组织的微流模型可以降低风险,但该技术仍主要依靠终点检测和生物标记物测量来评估组织损伤,从而导致有限的机械信息和难以检测低于阈值的不良反应。细胞损伤。在这里,我们介绍了一种传感器集成的肝芯片阵列,其中使用组织嵌入的微探针的两频相位调制来监测氧气,而使用微流电化学传感器实时测量葡萄糖,乳酸和温度。我们的微生理平台可以计算围绕中央碳代谢的代谢通量的动态变化,产生肝脏对刺激反应的独特代谢指纹。使用我们的平台,我们研究了人类肝脏对癫痫药物丙戊酸盐(Depakine™)和抗逆转录病毒药物Stavudine(Zerit™)的反应。使用E6 / E7LOW肝细胞,我们分别显示TC50为2.5和0.8 mM,并在2D和3D培养物中显着诱导脂肪变性。发病时间分析显示,缓慢的渐进性损伤仅在暴露后15-20小时开始。然而,通量分析显示代谢稳态的快速破坏发生在细胞损伤阈值以下。丙戊酸暴露导致脂肪生成持续增加15%,随后发生线粒体应激,而Stavudine暴露仅显示脂质生成短暂增加,表明β-氧化被破坏。
更新日期:2018-07-02
中文翻译:

微生理通量平衡平台揭示了药物性脂肪变性的动力学。
目前,动物实验无法准确预测人类的反应,阻碍了药物的研发。尽管新兴的器官上芯片技术使用人体组织的微流模型可以降低风险,但该技术仍主要依靠终点检测和生物标记物测量来评估组织损伤,从而导致有限的机械信息和难以检测低于阈值的不良反应。细胞损伤。在这里,我们介绍了一种传感器集成的肝芯片阵列,其中使用组织嵌入的微探针的两频相位调制来监测氧气,而使用微流电化学传感器实时测量葡萄糖,乳酸和温度。我们的微生理平台可以计算围绕中央碳代谢的代谢通量的动态变化,产生肝脏对刺激反应的独特代谢指纹。使用我们的平台,我们研究了人类肝脏对癫痫药物丙戊酸盐(Depakine™)和抗逆转录病毒药物Stavudine(Zerit™)的反应。使用E6 / E7LOW肝细胞,我们分别显示TC50为2.5和0.8 mM,并在2D和3D培养物中显着诱导脂肪变性。发病时间分析显示,缓慢的渐进性损伤仅在暴露后15-20小时开始。然而,通量分析显示代谢稳态的快速破坏发生在细胞损伤阈值以下。丙戊酸暴露导致脂肪生成持续增加15%,随后发生线粒体应激,而Stavudine暴露仅显示脂质生成短暂增加,表明β-氧化被破坏。











































 京公网安备 11010802027423号
京公网安备 11010802027423号