当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Is There a Precipitation Sequence in Municipal Wastewater Induced by Electrolysis?
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-07-17 , DOI: 10.1021/acs.est.8b02869 Yang Lei 1, 2 , Jorrit Christiaan Remmers 2 , Michel Saakes 1 , Renata D. van der Weijden 1, 2 , Cees J. N. Buisman 1, 2
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-07-17 , DOI: 10.1021/acs.est.8b02869 Yang Lei 1, 2 , Jorrit Christiaan Remmers 2 , Michel Saakes 1 , Renata D. van der Weijden 1, 2 , Cees J. N. Buisman 1, 2
Affiliation
|
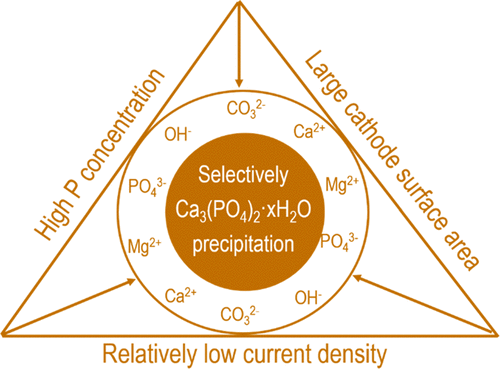
Electrochemical wastewater treatment can induce calcium phosphate precipitation on the cathode surface. This provides a simple yet efficient way for extracting phosphorus from municipal wastewater without dosing chemicals. However, the precipitation of amorphous calcium phosphate (ACP) is accompanied by the precipitation of calcite (CaCO3) and brucite (Mg(OH)2). To increase the content of ACP in the products, it is essential to understand the precipitation sequence of ACP, calcite, and brucite in electrochemical wastewater treatment. Given the fact that calcium phosphate (i.e., hydroxyapatite) has the lowest thermodynamic solubility product and highest saturation index in the wastewater, it has the potential to precipitate first. However, this is not observed in electrochemical phosphate recovery from raw wastewater, which is probably because of the very high Ca/P molar ratio (7.5) and high bicarbonate concentration in the wastewater resulting in formation of calcite. In the case of decreased Ca/P molar ratio (1.77) by spiking external phosphate, most of the removed Ca in the wastewater was used for ACP formation instead of calcite. The formation of of brucite, however, was only affected when the current density was decreased or the size of cathode was changed. Overall, the removal of Ca and Mg is much more affected by current density than the surface area of cathode, whereas for P removal, the reverse is true. Because of these dependencies, though there is no definite precipitation sequence among ACP, calcite, and brucite, it is still possible to influence the precipitation degree of these species by relatively low current density and high surface area or by targeting phosphorus-rich wastewaters.
中文翻译:

电解引起的城市废水中有沉淀序列吗?
电化学废水处理可导致磷酸钙沉淀在阴极表面。这提供了一种简单而有效的方法,无需添加化学药品即可从市政废水中提取磷。然而,无定形磷酸钙(ACP)的沉淀伴随有方解石(CaCO 3)和水镁石(Mg(OH)2的沉淀)。为了增加产品中ACP的含量,必须了解ACP,方解石和水镁石在电化学废水处理中的沉淀顺序。考虑到磷酸钙(即羟基磷灰石)在废水中具有最低的热力学溶解度产物和最高的饱和指数这一事实,它有可能首先沉淀。但是,这在从原废水中回收磷酸盐的电化学方法中未观察到,这可能是由于Ca / P摩尔比非常高(7.5)和废水中碳酸氢盐的浓度很高,导致了方解石的形成。如果通过添加外部磷酸盐而降低Ca / P摩尔比(1.77),则废水中去除的大部分Ca均用于ACP形成,而不是方解石。水镁石的形成,仅当电流密度降低或阴极尺寸改变时,才受到影响。总的来说,钙和镁的去除受电流密度的影响要比阴极的表面积大得多,而磷的去除则相反。由于这些依赖性,尽管ACP,方解石和水镁石之间没有确定的沉淀顺序,但仍可能通过相对较低的电流密度和较高的表面积或以富磷废水为目标来影响这些物种的沉淀程度。
更新日期:2018-07-18
中文翻译:

电解引起的城市废水中有沉淀序列吗?
电化学废水处理可导致磷酸钙沉淀在阴极表面。这提供了一种简单而有效的方法,无需添加化学药品即可从市政废水中提取磷。然而,无定形磷酸钙(ACP)的沉淀伴随有方解石(CaCO 3)和水镁石(Mg(OH)2的沉淀)。为了增加产品中ACP的含量,必须了解ACP,方解石和水镁石在电化学废水处理中的沉淀顺序。考虑到磷酸钙(即羟基磷灰石)在废水中具有最低的热力学溶解度产物和最高的饱和指数这一事实,它有可能首先沉淀。但是,这在从原废水中回收磷酸盐的电化学方法中未观察到,这可能是由于Ca / P摩尔比非常高(7.5)和废水中碳酸氢盐的浓度很高,导致了方解石的形成。如果通过添加外部磷酸盐而降低Ca / P摩尔比(1.77),则废水中去除的大部分Ca均用于ACP形成,而不是方解石。水镁石的形成,仅当电流密度降低或阴极尺寸改变时,才受到影响。总的来说,钙和镁的去除受电流密度的影响要比阴极的表面积大得多,而磷的去除则相反。由于这些依赖性,尽管ACP,方解石和水镁石之间没有确定的沉淀顺序,但仍可能通过相对较低的电流密度和较高的表面积或以富磷废水为目标来影响这些物种的沉淀程度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号