当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C-Methylation Catalyzed by Fom3, a Cobalamin-Dependent Radical S-adenosyl-l-methionine Enzyme in Fosfomycin Biosynthesis, Proceeds with Inversion of Configuration
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.biochem.8b00614 Shusuke Sato 1 , Fumitaka Kudo 1 , Tomohisa Kuzuyama 2 , Friedrich Hammerschmidt 3 , Tadashi Eguchi 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.biochem.8b00614 Shusuke Sato 1 , Fumitaka Kudo 1 , Tomohisa Kuzuyama 2 , Friedrich Hammerschmidt 3 , Tadashi Eguchi 1
Affiliation
|
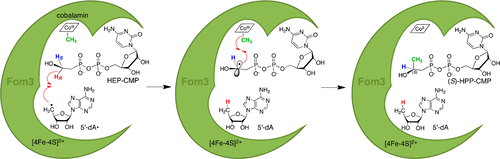
Fom3, a cobalamin-dependent radical S-adenosyl-l-methionine (SAM) methyltransferase, catalyzes C-methylation at the C2 position of cytidylylated 2-hydroxyethylphosphonate (HEP-CMP) to afford cytidylylated 2-hydroxypropylphosphonate (HPP-CMP) in fosfomycin biosynthesis. In this study, the Fom3 reaction product HPP-CMP was reanalyzed by chiral ligand exchange chromatography to confirm its stereochemistry. The Fom3 methylation product was found to be (S)-HPP-CMP only, indicating that the stereochemistry of the C-methylation catalyzed by Fom3 is (S)-selective. In addition, Fom3 reaction was performed with (S)-[2-2H1]HEP-CMP and (R)-[2-2H1]HEP-CMP to elucidate the stereoselectivity during the abstraction of the hydrogen atom from C2 of HEP-CMP. Liquid chromatography–electrospray ionization mass spectrometry analysis of the 5′-deoxyadenosine produced showed that the 2H atom of (R)-[2-2H1]HEP-CMP was incorporated into 5′-deoxyadenosine but that from (S)-[2-2H1]HEP-CMP was not. Retention of the 2H atom of (S)-[2-2H1]HEP-CMP in HPP-CMP was also observed. These results indicate that the 5′-deoxyadenosyl radical stereoselectively abstracts the pro-R hydrogen atom at the C2 position of HEP-CMP and the substrate radical intermediate reacts with the methyl group on cobalamin that is located on the opposite side of the substrate from SAM. Consequently, it was clarified that the C-methylation catalyzed by Fom3 proceeds with inversion of configuration.
中文翻译:

磷霉素生物合成中钴胺素依赖性自由基S-腺苷-1-蛋氨酸酶Fom3催化C-甲基化,构型反转
钴胺素依赖性自由基S-腺苷-1-甲硫氨酸(SAM)甲基转移酶Fom3催化胞嘧啶化的2-羟乙基膦酸酯(HEP-CMP)C2位置处的C-甲基化,从而提供磷霉素中的胞嘧啶化的2-羟丙基膦酸酯(HPP-CMP)生物合成。在这项研究中,通过手性配体交换色谱法重新分析了Fom3反应产物HPP-CMP,以确认其立体化学。发现该Fom3甲基化产物仅为(S)-HPP-CMP,表明由Fom3催化的C-甲基化的立体化学是(S)-选择性的。另外,用(S)-[2- 2 H 1进行了Fom3反应。] HEP-CMP和(R)-[2- 2 H 1 ] HEP-CMP,以阐明从HEP-CMP的C2提取氢原子时的立体选择性。产生的5'-脱氧腺苷的液相色谱-电喷雾电离质谱分析表明,(R)-[2- 2 H 1 ] HEP-CMP的2 H原子被并入5'-脱氧腺苷,但从(S)-没有[2- 2 H 1 ] HEP-CMP。(S)-[2- 2 H 1的2 H原子的保留在HPP-CMP中也观察到] HEP-CMP。这些结果表明5'-脱氧腺苷基团立体选择性地提取了HEP-CMP C2位置的pro-R氢原子,并且底物自由基中间体与钴胺素上的甲基反应,钴胺素位于与SAM相反的底物上。因此,澄清了由Fom3催化的C-甲基化随着构型的转化而进行。
更新日期:2018-07-02
中文翻译:

磷霉素生物合成中钴胺素依赖性自由基S-腺苷-1-蛋氨酸酶Fom3催化C-甲基化,构型反转
钴胺素依赖性自由基S-腺苷-1-甲硫氨酸(SAM)甲基转移酶Fom3催化胞嘧啶化的2-羟乙基膦酸酯(HEP-CMP)C2位置处的C-甲基化,从而提供磷霉素中的胞嘧啶化的2-羟丙基膦酸酯(HPP-CMP)生物合成。在这项研究中,通过手性配体交换色谱法重新分析了Fom3反应产物HPP-CMP,以确认其立体化学。发现该Fom3甲基化产物仅为(S)-HPP-CMP,表明由Fom3催化的C-甲基化的立体化学是(S)-选择性的。另外,用(S)-[2- 2 H 1进行了Fom3反应。] HEP-CMP和(R)-[2- 2 H 1 ] HEP-CMP,以阐明从HEP-CMP的C2提取氢原子时的立体选择性。产生的5'-脱氧腺苷的液相色谱-电喷雾电离质谱分析表明,(R)-[2- 2 H 1 ] HEP-CMP的2 H原子被并入5'-脱氧腺苷,但从(S)-没有[2- 2 H 1 ] HEP-CMP。(S)-[2- 2 H 1的2 H原子的保留在HPP-CMP中也观察到] HEP-CMP。这些结果表明5'-脱氧腺苷基团立体选择性地提取了HEP-CMP C2位置的pro-R氢原子,并且底物自由基中间体与钴胺素上的甲基反应,钴胺素位于与SAM相反的底物上。因此,澄清了由Fom3催化的C-甲基化随着构型的转化而进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号