Catalysis Today ( IF 5.2 ) Pub Date : 2018-07-02 , DOI: 10.1016/j.cattod.2018.06.056 Yuta Nakasaka , Takumi Kanda , Ken-ichi Shimizu , Kenichi Kon , Gen Shibata , Takao Masuda
|
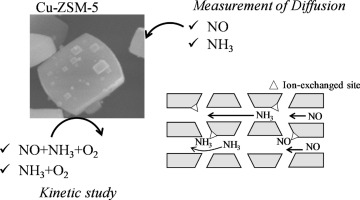
Micropore diffusivities of NO and NH3 were experimentally measured for Cu-ZSM-5 using constant volumetric method at a temperature range from 373 K to 423 K. Intracrystalline diffusivities and effective diffusivities of NO were lower than those of NH3, and the activation energy for intracrystalline diffusivity of NO (21 kJ mol−1) was higher than that of NH3 (6.3 kJ mol−1). The intracrystalline diffusivity of NO in Cu-ZSM-5 catalysts at 373 K was independent of Cu content and Si/Al ratio of Cu-ZSM-5. We proposed an equation for calculation of effective diffusivity of NO in Cu-ZSM-5 which was validated by the experimental data. Kinetic studies for NH3-SCR and NH3 oxidation were carried out using Cu-ZSM-5 with different crystal thickness. The apparent reaction rate of NH3-SCR at 523 K depended on the zeolite crystal thickness; the rates by a small Cu-ZSM-5 (0.088 μm) was 2.75 times higher than that by a large crystal catalyst (2.7 μm), corresponding to the effectiveness factor of 0.36. The apparent reaction rate of NH3 oxidation did not depend on the zeolite crystal size. It is concluded that NH3-SCR is controlled by intracrystalline diffusion of NO, or in other words, the active sites in zeolite crystal are not fully utilized due to the NO diffusion limitation. The effective diffusivity of NO in Cu-ZSM-5 estimated by the catalytic study was close to that calculated using the NO diffusivity results, which supported our conclusion.
中文翻译:

NO和NH 3在Cu-ZSM-5中的微孔扩散性及其对NH 3 -SCR的影响
NO和NH的微孔扩散系数3对Cu-ZSM-5进行了实验测量使用恒定的体积方法在温度范围从373 K至423个K.晶内扩散率和NO的有效扩散率明显高于NH的下部3,且活化能NO(21kJ mol -1)的晶内扩散率高于NH 3(6.3kJ mol -1)。在373 K下,NO在Cu-ZSM-5催化剂中的晶内扩散率与Cu-ZSM-5的Cu含量和Si / Al比无关。我们提出了一个计算NO-在Cu-ZSM-5中的有效扩散系数的方程,该方程已通过实验数据验证。NH 3 -SCR和NH 3的动力学研究使用具有不同晶体厚度的Cu-ZSM-5进行氧化。NH 3 -SCR在523 K时的表观反应速率取决于沸石的晶体厚度;较小的Cu-ZSM-5(0.088μm)的转化率比较大的晶体催化剂(2.7μm)的转化率高2.75倍,相当于有效系数0.36。NH 3氧化的表观反应速率不取决于沸石晶体的尺寸。结论是NH 3-SCR通过NO的晶内扩散来控制,或者换句话说,由于NO扩散的限制,不能充分利用沸石晶体中的活性位。催化研究估计的NO在Cu-ZSM-5中的有效扩散率接近于使用NO扩散率计算得出的结果,这支持了我们的结论。











































 京公网安备 11010802027423号
京公网安备 11010802027423号