当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitramino-functionalized tetracyclic oxadiazoles as energetic materials with high performance and high stability: crystal structures and energetic properties†
CrystEngComm ( IF 2.6 ) Pub Date : 2018-06-30 00:00:00 , DOI: 10.1039/c8ce00857d Qi Sun 1, 2, 3, 4 , Qiuhan Lin 1, 2, 3, 4 , Ming Lu 1, 2, 3, 4
CrystEngComm ( IF 2.6 ) Pub Date : 2018-06-30 00:00:00 , DOI: 10.1039/c8ce00857d Qi Sun 1, 2, 3, 4 , Qiuhan Lin 1, 2, 3, 4 , Ming Lu 1, 2, 3, 4
Affiliation
|
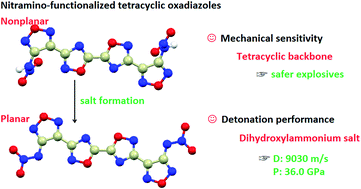
A series of tetracyclic energetic materials were developed through the introduction of 1,2,4-oxadiazole into nitramino-1,2,5-oxadiazole. All these thirteen new compounds were fully characterized and seven of them were further confirmed by single crystal X-ray diffraction. Salt formation led to planarization of the parent structure, reduction in lengths, increase in bond-dissociation energies of N–NO2 bonds, and formation of hydrogen bonds, which significantly increased decomposition temperatures from 84 °C (the neutral compound) to 187–303 °C (the energetic salts). Thus, the increase in thermal stability falls in the range of 103–219 °C. To the best of our knowledge, 219 °C is the largest reported increase in thermal stability due to salt formation. In addition, electrostatic surface potentials and noncovalent interactions (π–π stacking) were analyzed to understand structure–property relationships of these compounds. Both theoretical calculations and practical explosive experiments indicate that the dihydroxylammonium salt exhibits better detonation performance than the powerful explosive RDX. The tetracyclic backbone provides these compounds with enhanced stability. Short synthesis steps, low cost, good thermostability, excellent detonation performance, and acceptable mechanical sensitivity highlight the practical applications of these energetic compounds.
中文翻译:

高性能,高稳定性的高能材料硝化氨基官能化的四环恶二唑:晶体结构和高能性能†
通过将1,2,4-恶二唑引入硝基氨基-1,2,5-恶二唑中,开发了一系列四环含能材料。所有这13种新化合物均得到了充分表征,其中7种通过单晶X射线衍射进一步证实。盐的形成导致母体结构平面化,长度减少,N–NO 2的键解离能增加键和氢键的形成,将分解温度从84°C(中性化合物)显着提高到187–303°C(高能盐)。因此,热稳定性的增加落在103–219°C的范围内。据我们所知,219°C是由于形成盐引起的热稳定性增加的最大记录。此外,还分析了静电表面电势和非共价相互作用(π-π堆积)以了解这些化合物的结构-性质关系。理论计算和实际爆炸实验均表明,二羟基铵盐的爆炸性能优于强力炸药RDX。四环主链为这些化合物提供了增强的稳定性。合成步骤短,成本低,热稳定性好,
更新日期:2018-06-30
中文翻译:

高性能,高稳定性的高能材料硝化氨基官能化的四环恶二唑:晶体结构和高能性能†
通过将1,2,4-恶二唑引入硝基氨基-1,2,5-恶二唑中,开发了一系列四环含能材料。所有这13种新化合物均得到了充分表征,其中7种通过单晶X射线衍射进一步证实。盐的形成导致母体结构平面化,长度减少,N–NO 2的键解离能增加键和氢键的形成,将分解温度从84°C(中性化合物)显着提高到187–303°C(高能盐)。因此,热稳定性的增加落在103–219°C的范围内。据我们所知,219°C是由于形成盐引起的热稳定性增加的最大记录。此外,还分析了静电表面电势和非共价相互作用(π-π堆积)以了解这些化合物的结构-性质关系。理论计算和实际爆炸实验均表明,二羟基铵盐的爆炸性能优于强力炸药RDX。四环主链为这些化合物提供了增强的稳定性。合成步骤短,成本低,热稳定性好,











































 京公网安备 11010802027423号
京公网安备 11010802027423号