当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Efficient Removal of Uranium from Aqueous Solution Using a Magnetic Adsorbent Bearing Phosphine Oxide Ligand: A Combined Experimental and Density Functional Theory Study
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-06-30 00:00:00 , DOI: 10.1021/acssuschemeng.7b04352 Dingzhong Yuan 1, 2 , Shiao Zhang 2 , Zhihao Xiang 2 , Yan Liu 2 , Yun Wang 3 , Xinyue Zhou 2 , Yan He 2 , Wenjun Huang 2 , Qinghua Zhang 2
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-06-30 00:00:00 , DOI: 10.1021/acssuschemeng.7b04352 Dingzhong Yuan 1, 2 , Shiao Zhang 2 , Zhihao Xiang 2 , Yan Liu 2 , Yun Wang 3 , Xinyue Zhou 2 , Yan He 2 , Wenjun Huang 2 , Qinghua Zhang 2
Affiliation
|
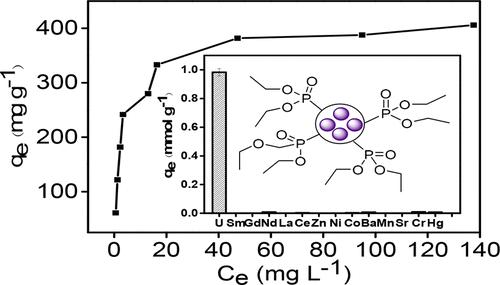
It is still a great challenge to develop magnetic adsorbents for the highly efficient entrapment of uranium from aqueous solution. Herein, a novel magnetic adsorbent (denoted as Fe3O4/P (AA-MMA-DVP)) bearing phosphine oxide ligand was designed and synthesized via a DPE (1,1-diphenylethylene) method based on DPE as radical controlling agent, showing an excellent adsorption capacity for uranium at pH 4.5 and outstanding selectivity in aqueous media including 14 coexisting ions. The magnetic adsorbent showed a qmax value of 413.2 mg g–1 at 298 K and pH 4.5, which was higher than that of most of other magnetic adsorbents. The outstanding selectivity (Su = 95.8%) for uranium was reasonably ascribed to the strong complexation between UO22+ and P═O groups anchored on the polymer skeletion, which was evidenced by experimental results. Furthermore, the magnetic adsorbent could be isolated by magnetic force and be recycled at least five times without significant loss in adsorption capacity. This work provided a convenient synthetic route to develop a novel magnetic adsorbent with high capacity and strong selectivity for the entrapment of uranium from aqueous solution.
中文翻译:

含磷氧化物配体的磁性吸附剂从水溶液中高效去除铀的实验与密度泛函理论的结合
开发用于从水溶液中高效截留铀的磁性吸附剂仍然是一个巨大的挑战。在此,以DPE为自由基控制剂,采用DPE(1,1-二苯基乙烯)法设计并合成了一种新型的带有膦氧化物配体的磁性吸附剂(Fe 3 O 4 / P(AA-MMA-DVP)),在pH 4.5下对铀具有出色的吸附能力,在包括14种共存离子的水性介质中具有出色的选择性。在298 K和pH值为4.5时,磁性吸附剂的q最大值为413.2 mg g –1,高于大多数其他磁性吸附剂的q最大值。出色的选择性(S u铀含量为95.8%)合理地归因于UO 2 2+与锚定在聚合物骨架上的P═O基团之间的强络合作用,实验结果证明了这一点。此外,磁性吸附剂可以通过磁力分离并且可以循环至少五次而不会显着降低吸附能力。这项工作为开发一种新型的磁性吸附剂提供了便利的合成途径,该吸附剂具有高容量和强选择性,可从水溶液中截留铀。
更新日期:2018-06-30
中文翻译:

含磷氧化物配体的磁性吸附剂从水溶液中高效去除铀的实验与密度泛函理论的结合
开发用于从水溶液中高效截留铀的磁性吸附剂仍然是一个巨大的挑战。在此,以DPE为自由基控制剂,采用DPE(1,1-二苯基乙烯)法设计并合成了一种新型的带有膦氧化物配体的磁性吸附剂(Fe 3 O 4 / P(AA-MMA-DVP)),在pH 4.5下对铀具有出色的吸附能力,在包括14种共存离子的水性介质中具有出色的选择性。在298 K和pH值为4.5时,磁性吸附剂的q最大值为413.2 mg g –1,高于大多数其他磁性吸附剂的q最大值。出色的选择性(S u铀含量为95.8%)合理地归因于UO 2 2+与锚定在聚合物骨架上的P═O基团之间的强络合作用,实验结果证明了这一点。此外,磁性吸附剂可以通过磁力分离并且可以循环至少五次而不会显着降低吸附能力。这项工作为开发一种新型的磁性吸附剂提供了便利的合成途径,该吸附剂具有高容量和强选择性,可从水溶液中截留铀。



























 京公网安备 11010802027423号
京公网安备 11010802027423号