Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-06-30 , DOI: 10.1016/j.bmc.2018.06.038 Lorena Navarro , Gloria Rosell , Silvia Sánchez , Núria Boixareu , Klaus Pors , Ramon Pouplana , Josep M. Campanera , M. Dolors Pujol
|
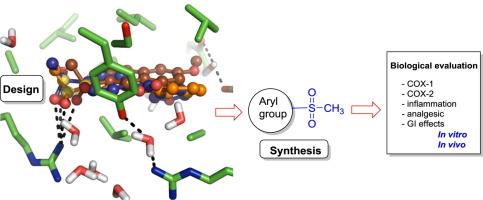
A novel group of aryl methyl sulfones based on nonsteroidal anti-inflammatory compounds exhibiting a methyl sulfone instead of the acetic or propionic acid group was designed, synthesized and evaluated in vitro for inhibition against the human cyclooxygenase of COX-1 and COX-2 isoenzymes and in vivo for anti-inflammatory activity using the carrageenan induced rat paw edema model in rats. Also, in vitro chemosensitivity and in vivo analgesic and intestinal side effects were determined for defining the therapeutic and safety profile. Molecular modeling assisted the design of compounds and the interpretation of the experimental results. Biological assay results showed that methyl sulfone compounds 2 and 7 were the most potent COX inhibitors of this series and best than the corresponding carboxylic acids (methyl sulfone 2: IC50 COX-1 = 0.04 and COX-2 = 0.10 μM, and naproxen: IC50 COX-1 = 11.3 and COX-2 = 3.36 μM). Interestingly, the inhibitory activity of compound 2 represents a significant improvement compared to that of the parent carboxylic compound, naproxen. Further support to the results were gained by the docking studies which suggested the ability of compound 2 and 7 to bind into COX enzyme with low binding free energies.
The improvement of the activity of some sulfones compared to the carboxylic analogues would be performed through a change of the binding mode or mechanism compared to the standard binding mode displayed by ibuprofen, as disclosed by molecular modeling studies. So, this study paves the way for further attention in investigating the participation of these new compounds in the pain inhibitory mechanisms. The most promising compounds 2 and 7 possess a therapeutical profile that enables their chemical scaffolds to be utilized for development of new NSAIDs.
中文翻译:

芳基甲基砜的合成及生物学性质
设计,合成和评估了一组基于非甾体抗炎化合物的新型芳基甲基砜,该化合物显示了甲基砜而不是乙酸或丙酸基团,并在体外进行了评估,以抑制人环氧化酶对COX-1和COX-2同工酶和角叉菜胶诱导的大鼠爪水肿模型在体内的抗炎活性。此外,确定了体外化学敏感性以及体内止痛和肠道副作用以定义治疗和安全性。分子建模有助于化合物的设计和实验结果的解释。生物测定结果表明,甲基砜化合物2和7种是该系列中最有效的COX抑制剂,并且比相应的羧酸类最好(甲基砜2:IC 50 COX-1 = 0.04和COX-2 = 0.10μM,萘普生:IC 50 COX-1 = 11.3和COX- 2 = 3.36μM)。有趣的是,与母体羧酸化合物萘普生相比,化合物2的抑制活性显着提高。通过对接研究获得了对结果的进一步支持,对接研究表明化合物2和7具有以低结合自由能结合到COX酶中的能力。
如分子模型研究所公开的,与羧酸类似物相比,某些砜的活性的改善将通过改变与布洛芬显示的标准结合模式相比的结合模式或机理来进行。因此,本研究为进一步研究这些新化合物在疼痛抑制机制中的参与铺平了道路。最有前途的化合物2和7具有使它们的化学支架能够用于开发新的NSAID的治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号