Bioelectrochemistry ( IF 4.8 ) Pub Date : 2018-06-25 , DOI: 10.1016/j.bioelechem.2018.06.006 Izabella Brand , Karl-Wilhelm Koch
|
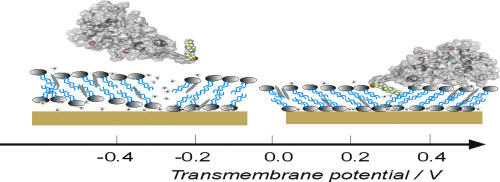
The neuronal calcium sensor protein recoverin is expressed in retinal rod and cone cells and is involved in the calcium-dependent control of receptor (rhodopsin) phosphorylation and receptor inactivation. In its Ca2+-saturated form recoverin is attached to membranes by an exposed myristoyl group and responds to oscillating changes of intracellular Ca2+-concentration by performing a so-called Ca2+-myristoyl switch. In this work we analyze changes in a liquid lipid bilayer interacting with myristoylated and non-myristoylated recoverin by employing polarization modulation infrared reflection absorption spectroscopy (PM IRRAS) with electrochemical control. The lipid bilayer is transferred onto a polycrystalline gold electrode using Langmuir-Blodgett Langmuir-Schaefer transfer at the surface pressure π = 30 mN m−1 which ensures, necessary for the lipid-protein interaction, liquid state of the hydrocarbon chains of phospholipids. The model lipid bilayers are adsorbed directly on the Au electrode surface at transmembrane potentials −0.2 < ∆ϕM|S < 0.25 V. The interaction with recoverin leads to a stabilization of the adsorbed state of the lipid bilayer at positive transmembrane potentials. The interaction leads to a decrease in the surface charge density that accumulates on the membrane covered electrode surface, indicating changes in the lateral interactions in the lipid membrane. In situ spectroelectrochemical studies confirm orientation changes in the hydrophobic environment of the model membrane. Insertion of the myristoyl group of recoverin into the membrane is connected with an increase in the tilt of the hydrocarbon chains with respect to the surface normal and decrease in the bilayer thickness. Potential-induced pore formation and desorption of the lipid bilayer from the membrane surface is accompanied by the removal of the acyl chains of recoverin from the membrane.
中文翻译:

蛋白质肉豆蔻酰化对处于蛋白质结合状态的模型细胞膜结构的影响
神经钙传感器蛋白retinin在视网膜杆和视锥细胞中表达,并参与钙依赖的受体(视紫红质)磷酸化和受体失活的控制。在其Ca 2+饱和形式下,recoverin通过暴露的肉豆蔻酰基团附着在膜上,并通过执行所谓的Ca 2+来响应细胞内Ca 2 +-浓度的振荡变化。-肉豆蔻酰开关。在这项工作中,我们通过采用电化学控制的偏振调制红外反射吸收光谱(PM IRRAS),分析了与肉豆蔻酰化和非肉豆蔻酰化的恢复蛋白相互作用的液体脂质双层的变化。使用Langmuir-Blodgett Langmuir-Schaefer转移在表面压力π= 30 mN m -1下将脂质双层转移到多晶金电极上对于脂质-蛋白质相互作用而言,这确保了磷脂烃链的液态。模型脂质双层在跨膜电位-0.2 <∆M | S <0.25 V时直接吸附在Au电极表面上。与coverin的相互作用导致脂质双层在跨膜电位为正时的吸附状态稳定。相互作用导致积累在膜覆盖的电极表面上的表面电荷密度降低,表明脂质膜中的横向相互作用发生了变化。原位光谱电化学研究证实了模型膜疏水环境中的取向变化。恢复蛋白的肉豆蔻酰基插入膜中与烃链相对于表面法线的倾斜度增加和双层厚度的减少有关。潜在诱导的孔形成和脂质双层从膜表面的解吸伴随着从膜上去除回收蛋白的酰基链。











































 京公网安备 11010802027423号
京公网安备 11010802027423号