Organic Electronics ( IF 2.7 ) Pub Date : 2018-06-30 , DOI: 10.1016/j.orgel.2018.06.042 Xiang Yao , Wei Shao , Xuan Xiang , Wen-Jing Xiao , Long Liang , Fu-Gang Zhao , Jun Ling , Zhengquan Lu , Jingjing Li , Wei-Shi Li
|
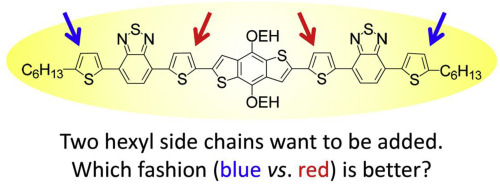
Small molecular (SM) semiconductors with sufficient solubility and high performance are highly desired in solution-processed organic electronics. In order to address the poor solubility that a reported high performance molecular semiconductor (BDT(ThBTTh)2) with a benzodithiophene (BDT) core and two thiophene-benzothiadiazole-thiophene arms (ThBTTh) suffers from, two additional hexyl side chains have been designed into its scaffold and different attaching fashions yield two new SM semiconductors, SM1 and SM2. The integration positions in SM1 are terminal thiophene units, while that of SM2 are located on the inner thiophene units neighbor to the central BDT unit. With expectation, the so-prepared SM1 and SM2 possess much better solubility than BDT(ThBTTh)2. However, the integration of additional alkyl side chains sacrifices its optoelectronic performance and the sacrifice extent is highly dependent on their attaching fashions. The molecule SM2 having new additional side chains in two inner thiophene units displayed much better performance (hole mobility: 1.80 × 10−2 cm2 V-1 s-1, solar cell efficiency: (2.57 ± 0.17)%) than SM1 that integrates them at the two terminal thiophene units (hole mobility: 2.18 × 10−4 cm2 V-1 s-1, solar cell efficiency: (0.87 ± 0.11)%). For understanding the origination of such performance differences, detail characterizations including differential scanning calorimetry, ultraviolet–visible absorption spectroscopy, cyclic voltammetry, density function theoretical calculation, X-ray diffraction and atomic force microscopy have been carried out. Finally, benefiting from their enhanced good solubility in non-chlorinated solvents, toluene has been proved capable as a green processing solvent for organic solar cell fabrication. The so-obtained SM2 devices displayed an optimized efficiency of (2.35 ± 0.19)%.
中文翻译:

小分子半导体上的侧链工程:通过为烷基侧链选择合适的位置,在溶解度和性能之间取得平衡
在溶液处理的有机电子产品中,高度希望具有足够的溶解度和高性能的小分子(SM)半导体。为了解决报告的具有苯并二噻吩(BDT)核心和两个噻吩-苯并噻二唑-噻吩臂(ThBTTh)的高性能分子半导体(BDT(ThBTTh)2)所遭受的不良溶解性,已设计了两个附加的己基侧链进入其脚手架并以不同的附着方式产生了两种新的SM半导体,SM1和SM2。SM1中的整合位置是末端噻吩单元,而SM2中的整合位置它们位于与中央BDT单元相邻的内部噻吩单元上。可以预期,如此制备的SM1和SM2具有比BDT(ThBTTh)2更好的溶解度。然而,额外烷基侧链的整合牺牲了其光电性能,并且牺牲程度高度依赖于它们的附着方式。在两个内部噻吩单元中具有新的侧链的分子SM2显示出比SM1更好的性能(空穴迁移率:1.80×10 -2 cm 2 V -1 s -1,太阳能电池效率:(2.57±0.17)%)将它们整合在两个末端噻吩单元上(空穴迁移率:2.18×10 -4 cm 2 V -1 s -1,太阳能电池效率:(0.87±0.11)%)。为了理解这种性能差异的根源,已进行了详细的表征,包括差示扫描量热法,紫外可见吸收光谱法,循环伏安法,密度函数理论计算,X射线衍射和原子力显微镜。最后,受益于甲苯在非氯化溶剂中增强的良好溶解性,已证明甲苯能够用作有机太阳能电池制造的绿色加工溶剂。如此获得的SM2器件显示出(2.35±0.19)%的优化效率。









































 京公网安备 11010802027423号
京公网安备 11010802027423号