当前位置:
X-MOL 学术
›
Fuel Process. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of n-butanol oxidation over Pt/ZSM-5 catalyst
Fuel Processing Technology ( IF 7.2 ) Pub Date : 2018-10-01 , DOI: 10.1016/j.fuproc.2018.06.020 Weijuan Yang , Xing Zhang , Jiale Su , Yefeng Wang , Qingchen Zhao , Junhu Zhou
Fuel Processing Technology ( IF 7.2 ) Pub Date : 2018-10-01 , DOI: 10.1016/j.fuproc.2018.06.020 Weijuan Yang , Xing Zhang , Jiale Su , Yefeng Wang , Qingchen Zhao , Junhu Zhou
|
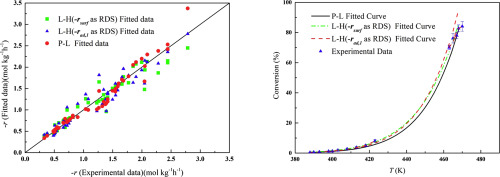
Abstract A kinetic study of n-butanol catalytic combustion over the Pt/ZSM-5 was performed in a fixed-bed combustor at ambient pressure and temperature range in 390–470 K. The Power Law model and Langmuir-Hinshelwood (L–H) model were constructed to characterize the n-butanol catalytic oxidation. The reaction orders of n-butanol and O2 were −0.24 and 1, respectively. Five pathways rate expressions were selected to predict the reaction rate for L–H model. The sum of the squared differences between the experimental data and the fitted data indicated that surface reaction and oxygen adsorption were probably rate-determining steps. The reliability of the rate expressions was affirmed by comparing the experimental and the fitted rates.
中文翻译:

Pt/ZSM-5催化剂上正丁醇氧化动力学
摘要 在固定床燃烧器中,在 390-470 K 的环境压力和温度范围内对 Pt/ZSM-5 上的正丁醇催化燃烧进行了动力学研究。幂律模型和 Langmuir-Hinshelwood (L-H)构建模型来表征正丁醇催化氧化。正丁醇和O2的反应级数分别为-0.24和1。选择了五种途径速率表达式来预测 L-H 模型的反应速率。实验数据和拟合数据之间的平方差之和表明表面反应和氧吸附可能是决定速率的步骤。通过比较实验率和拟合率,肯定了率表达式的可靠性。
更新日期:2018-10-01
中文翻译:

Pt/ZSM-5催化剂上正丁醇氧化动力学
摘要 在固定床燃烧器中,在 390-470 K 的环境压力和温度范围内对 Pt/ZSM-5 上的正丁醇催化燃烧进行了动力学研究。幂律模型和 Langmuir-Hinshelwood (L-H)构建模型来表征正丁醇催化氧化。正丁醇和O2的反应级数分别为-0.24和1。选择了五种途径速率表达式来预测 L-H 模型的反应速率。实验数据和拟合数据之间的平方差之和表明表面反应和氧吸附可能是决定速率的步骤。通过比较实验率和拟合率,肯定了率表达式的可靠性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号