当前位置:
X-MOL 学术
›
Chem. Phys. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The acid strength of the datively bound complexes involving AlF3 lone pair acceptor and various lone pair donors
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-06-30 , DOI: 10.1016/j.cplett.2018.06.059 Olimpia Rybacka , Jakub Brzeski , Iwona Anusiewicz , Piotr Skurski
中文翻译:

涉及AlF 3孤对受体和各种孤对供体的双键结合配合物的酸强度
更新日期:2018-07-01
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-06-30 , DOI: 10.1016/j.cplett.2018.06.059 Olimpia Rybacka , Jakub Brzeski , Iwona Anusiewicz , Piotr Skurski
|
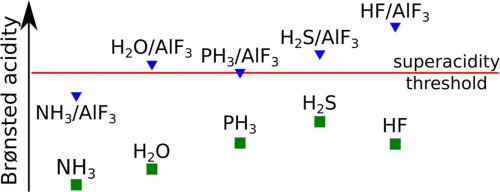
The acid strength of the datively bound X→AlF3 complexes (X=HF, HCl, H2S, AsH3, PH3, NF2H, NFH2, NH3, and H2O) is evaluated on the basis of theoretical calculations employing ab initio methods. Significant enhancement of the X acidity upon the formation of X/AlF3 compounds is predicted. It is demonstrated that even the non-acidic molecules X (e.g., H2O, NH3) combined with AlF3 are expected to form the X→AlF3 complexes characterized by the acid strength comparable or larger than that of H2SO4.
中文翻译:

涉及AlF 3孤对受体和各种孤对供体的双键结合配合物的酸强度
根据以下公式评估固定结合的X →AlF 3配合物(X = HF,HCl,H 2 S,AsH 3,PH 3,NF 2 H,NFH 2,NH 3和H 2 O)的酸强度。从头算方法进行理论计算。预计在形成X / AlF 3化合物时X酸度会显着提高。结果表明,甚至与AlF 3结合的非酸性分子X(例如H 2 O,NH 3)也会形成X →AlF 3络合物,其酸强度与H 2 SO 4相当或更大。



























 京公网安备 11010802027423号
京公网安备 11010802027423号