Catalysis Today ( IF 5.2 ) Pub Date : 2018-06-28 , DOI: 10.1016/j.cattod.2018.06.043 Jie Zhang , Chuang Li , Xiao Chen , Weixiang Guan , Changhai Liang
|
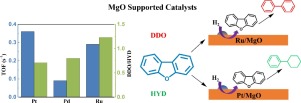
Conversion of oxygen-containing compounds derived from lignin arises wide interest due to fossil-derived resources consumption and growing environmental concerns. In this work, hydrodeoxygenation (HDO) of dibenzofuran (DBF) was studied over high-surface-area MgO supported Pt, Pd and Ru catalysts at 370 °C and 1.0 MPa. It was determined that the active metals not only affect the catalytic activity, but also change the reaction pathway of HDO of DBF. The intrinsic activity (TOF) of MgO supported catalysts follows the trend: Pt/MgO (0.36 s−1) > Ru/MgO (0.29 s−1) > Pd/MgO (0.09 s−1), companied by the increasing activate barrier. Pt has a high activity in the hydrogenation of DBF, and exhibits a perfect deoxygenation activity followed by hydrogenation (HYD) pathway. Ru shows better cleavage ability of CaromaticO bond and the removal of oxygen from DBF mainly occurs via direct deoxygenation (DDO) pathway. The increased Pt loadings largely promote the conversion of DBF by enhancing both HYD and DDO pathways. In addition, more direct cleavage of Caromatic
O bond occurs at higher temperature and the production of aromatics by the DDO pathway prefers the relatively low reaction pressure. Based on the pseudo-first-order kinetics, the analysis of the fitted reaction rate constant shows that the DDO selectivity follows the order: Ru/MgO > Pd/MgO > Pt/MgO, which depends on the capacity of active metals to the cleavage of Caromatic
O bond and the hydrogenation of aromatic ring.
中文翻译:

MgO负载贵金属催化剂上二苯并呋喃加氢脱氧反应途径的见解
由于源自化石的资源消耗和对环境的日益关注,衍生自木质素的含氧化合物的转化引起了广泛的关注。在这项工作中,在370°C和1.0 MPa的高表面积MgO负载的Pt,Pd和Ru催化剂上研究了二苯并呋喃(DBF)的加氢脱氧(HDO)。已确定活性金属不仅影响催化活性,而且改变了DBF的HDO的反应途径。MgO负载型催化剂的固有活性(TOF)遵循以下趋势:Pt / MgO(0.36 s -1)> Ru / MgO(0.29 s -1)> Pd / MgO(0.09 s -1),并伴有越来越多的激活障碍。Pt在DBF的氢化中具有很高的活性,并且表现出理想的脱氧活性,随后是氢化(HYD)途径。Ru表现出更好的C芳烃O键裂解能力,而从DBF中去除氧主要是通过直接脱氧(DDO)途径进行的。Pt负载的增加通过增强HYD和DDO途径大大促进了DBF的转化。此外,C芳烃的更直接裂解
O键在较高温度下发生,并且通过DDO途径产生芳族化合物优选相对较低的反应压力。基于拟一级反应动力学,拟合反应速率常数的分析表明,DDO的选择性遵循以下顺序:Ru / MgO> Pd / MgO> Pt / MgO,这取决于活性金属的裂解能力。 C芳族
O键的键合和芳族环的氢化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号