当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic Catalysis for the Umpolung Trifluoromethylthiolation of Tertiary Ethers
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-12 , DOI: 10.1002/anie.201805927 Wentao Xu 1 , Junyang Ma 1 , Xiang-Ai Yuan 2 , Jie Dai 1 , Jin Xie 1 , Chengjian Zhu 1, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-12 , DOI: 10.1002/anie.201805927 Wentao Xu 1 , Junyang Ma 1 , Xiang-Ai Yuan 2 , Jie Dai 1 , Jin Xie 1 , Chengjian Zhu 1, 3
Affiliation
|
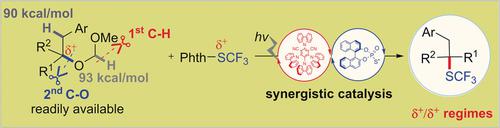
The first transition‐metal‐free, site‐specific umpolung trifluoromethylthiolation of tertiary alkyl ethers has been developed, achieving the challenging tertiary C(sp3)–SCF3 coupling under redox‐neutral conditions. The synergism of organophotocatalyst 4CzIPN and BINOL‐based phosphorothiols can site‐selectively cleave tertiary sp3 C(sp3)–O ether bonds in complex molecules initiated by a polarity‐matching hydrogen‐atom‐transfer (HAT) event. The incorporation of several competing benzylic and methine C(sp3)−H bonds in alkyl ethers has little influence on the regioselectivity. Selective difluoromethylthiolation of C−O bonds has also been achieved. This represents not only an important step forward in trifluoromethylthiolation but also a promising means for site‐selective C−O bond functionalization of unsymmetrical tertiary alkyl ethers.
中文翻译:

协同催化叔醚三氟甲基硫醇化
已经开发出第一个无过渡金属,特定位点的叔烷基醚的三氟甲基硫代三氟甲基硫醇化反应,实现了在氧化还原-中性条件下具有挑战性的叔C(sp 3)-SCF 3偶联。有机光催化剂4CzIPN和基于BINOL的硫代硫醇的协同作用可以在极性匹配的氢原子转移(HAT)事件引发的复杂分子中选择性地裂解叔sp 3 C(sp 3)-O醚键。几个竞争性苄基和次甲基C(sp 3的合并烷基醚中的)-H键对区域选择性影响很小。还已经实现了对CO键的选择性二氟甲基硫醇化。这不仅代表了三氟甲基硫醇化的重要一步,而且是不对称叔烷基醚的位点选择性C-O键功能化的一种有前途的手段。
更新日期:2018-07-12
中文翻译:

协同催化叔醚三氟甲基硫醇化
已经开发出第一个无过渡金属,特定位点的叔烷基醚的三氟甲基硫代三氟甲基硫醇化反应,实现了在氧化还原-中性条件下具有挑战性的叔C(sp 3)-SCF 3偶联。有机光催化剂4CzIPN和基于BINOL的硫代硫醇的协同作用可以在极性匹配的氢原子转移(HAT)事件引发的复杂分子中选择性地裂解叔sp 3 C(sp 3)-O醚键。几个竞争性苄基和次甲基C(sp 3的合并烷基醚中的)-H键对区域选择性影响很小。还已经实现了对CO键的选择性二氟甲基硫醇化。这不仅代表了三氟甲基硫醇化的重要一步,而且是不对称叔烷基醚的位点选择性C-O键功能化的一种有前途的手段。











































 京公网安备 11010802027423号
京公网安备 11010802027423号