Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-06-28 , DOI: 10.1016/j.jcat.2018.06.004 Dennis Vogelsang , Johanna Vondran , Andreas J. Vorholt
|
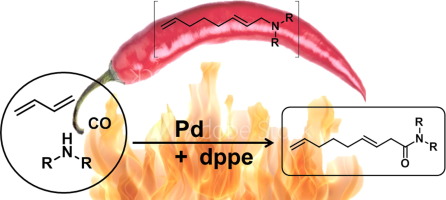
The first example of the palladium catalysed amidotelomerisation is presented, in style of the ambitious carboxytelomerisation. A straightforward synthetic tool was generated to produce several industrial relevant pelargonic C9-amides based on the fundamental chemical feedstocks: 1,3-butadiene, carbon monoxide and secondary amines. The reaction network was uncovered and crucial influences were determined by design of experiments (DoE). Through the incorporation of an auto-tandem palladium acetate/diphenylphosphino ethane catalytic system, very good yields up to 77% of the desired amides and excellent selectivities of carbonylation products of 94% were achieved. The application of the amidotelomerisation conditions to different classes of amines offered a broad range of the corresponding pelargonic C9-amides. Understanding the tandem catalysis, significant inhibition factors were uncovered and through a stepwise optimisation, for the first time a carbonylation reaction of octadienyl amines (telomer products) was shown, yielding in 99% of the desired linear pelargonic C9-amide.
中文翻译:

直接由1,3-丁二烯一步法钯催化合成不饱和壬烯基C 9-酰胺的方法
以雄心勃勃的羧基端粒化的形式,提出了钯催化的酰胺基端粒化的第一个例子。产生了一种简单的合成工具,可以基于基本的化学原料生产几种工业相关的壬基碳9酰胺:1,3-丁二烯,一氧化碳和仲胺。发现了反应网络,并通过实验设计(DoE)确定了关键影响。通过合并汽车-串联的乙酸钯/二苯基膦乙烷乙烷催化体系,获得了高达77%所需酰胺的非常高的收率,以及94%的优异羰基化产物选择性。将酰胺基端粒化条件应用于不同种类的胺提供了广泛的相应的壬基C 9-酰胺。了解串联催化后,发现了显着的抑制因子,并通过逐步优化,首次显示了辛二烯基胺(端粒产物)的羰基化反应,可制得99%的所需线性pelargonic C 9-酰胺。










































 京公网安备 11010802027423号
京公网安备 11010802027423号