当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dechlorination of Excess Trichloroethene by Bimetallic and Sulfidated Nanoscale Zero-Valent Iron
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-12 , DOI: 10.1021/acs.est.8b01735 Feng He 1, 2 , Zhenjie Li 1 , Shasha Shi 1 , Wenqiang Xu 1 , Hanzhen Sheng 1 , Yawei Gu 1 , Yonghai Jiang 3, 4 , Beidou Xi 3, 4
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-12 , DOI: 10.1021/acs.est.8b01735 Feng He 1, 2 , Zhenjie Li 1 , Shasha Shi 1 , Wenqiang Xu 1 , Hanzhen Sheng 1 , Yawei Gu 1 , Yonghai Jiang 3, 4 , Beidou Xi 3, 4
Affiliation
|
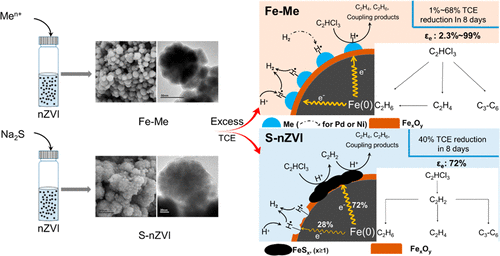
Nanoscale zerovalent iron (nZVI) likely finds its application in source zone remediation. Two approaches to modify nZVI have been reported: bimetal (Fe–Me) and sulfidated nZVI (S-nZVI). However, previous research has primarily focused on enhancing particle reactivity with these two modifications under more plume-like conditions. In this study, we systematically compared the trichloroethene (TCE) dechlorination pathway, rate, and electron selectivity of Fe-Me (Me: Pd, Ni, Cu, and Ag), S-nZVI, and nZVI with excess TCE simulating source zone conditions. TCE dechlorination on Fe-Me was primarily via hydrogenolysis while that on S-nZVI and nZVI was mainly via β-elimination. The surface-area normalized TCE reduction rate (k′SA) of Fe–Pd, S-nZVI, Fe–Ni, Fe–Cu, and Fe–Ag were ∼6800-, 190-, 130-, 20-, and 8-fold greater than nZVI. All bimetallic modification enhanced the competing hydrogen evolution reaction (HER) while sulfidation inhibited HER. Fe–Cu and Fe–Ag negligibly enhanced electron utilization efficiency (εe) while Fe–Pd, Fe–Ni, and S-nZVI dramatically increased εe from 2% to ∼100%, 69%, and 72%, respectively. Adsorbed atomic hydrogen was identified to be responsible for the TCE dechlorination on Fe–Me but not on S-nZVI. The enhanced dechlorination rate along with the reduced HER of S-nZVI can be explained by that FeS conducting major electrons mediated TCE dechlorination while Fe oxides conducting minor electrons mediated HER.
中文翻译:

双金属和硫化的纳米零价铁对过量三氯乙烯的脱氯
纳米级零价铁(nZVI)可能会在源区修复中找到其应用。已经报道了两种改性nZVI的方法:双金属(Fe-Me)和硫化nZVI(S-nZVI)。然而,先前的研究主要集中在在更类似于羽状的条件下通过这两种修饰来提高颗粒反应性。在这项研究中,我们系统地比较了Fe-Me(Me:Pd,Ni,Cu和Ag),S-nZVI和nZVI的三氯乙烯(TCE)脱氯途径,速率和电子选择性,并用过量的TCE模拟了源区条件。Fe-Me的TCE脱氯主要是通过氢解,而S-nZVI和nZVI的TCE脱氯主要是通过β-消除。表面区域归一化的TCE减少率(ķ ' SAFe-Pd,S-nZVI,Fe-Ni,Fe-Cu和Fe-Ag分别比nZVI大6800倍,190倍,130倍,20倍和8倍。所有双金属修饰均增强了竞争性放氢反应(HER),而硫化抑制了HER。的Fe-Cu和Fe基的Ag可忽略的增强的电子的利用效率(ε ë)而Fe-PD,Fe-Ni系,和S-的nZVI急剧增加ε ë分别从2%至约100%,69%和72%。吸附的氢原子被认为是导致Fe-Me上TCE脱氯的原因,而不是S-nZVI上的TCE脱氯。S-nZVI的脱氯速率提高,而HER降低,可以解释为FeS进行主要电子介导的TCE脱氯,而Fe氧化物进行次电子介导的HER。
更新日期:2018-07-14
中文翻译:

双金属和硫化的纳米零价铁对过量三氯乙烯的脱氯
纳米级零价铁(nZVI)可能会在源区修复中找到其应用。已经报道了两种改性nZVI的方法:双金属(Fe-Me)和硫化nZVI(S-nZVI)。然而,先前的研究主要集中在在更类似于羽状的条件下通过这两种修饰来提高颗粒反应性。在这项研究中,我们系统地比较了Fe-Me(Me:Pd,Ni,Cu和Ag),S-nZVI和nZVI的三氯乙烯(TCE)脱氯途径,速率和电子选择性,并用过量的TCE模拟了源区条件。Fe-Me的TCE脱氯主要是通过氢解,而S-nZVI和nZVI的TCE脱氯主要是通过β-消除。表面区域归一化的TCE减少率(ķ ' SAFe-Pd,S-nZVI,Fe-Ni,Fe-Cu和Fe-Ag分别比nZVI大6800倍,190倍,130倍,20倍和8倍。所有双金属修饰均增强了竞争性放氢反应(HER),而硫化抑制了HER。的Fe-Cu和Fe基的Ag可忽略的增强的电子的利用效率(ε ë)而Fe-PD,Fe-Ni系,和S-的nZVI急剧增加ε ë分别从2%至约100%,69%和72%。吸附的氢原子被认为是导致Fe-Me上TCE脱氯的原因,而不是S-nZVI上的TCE脱氯。S-nZVI的脱氯速率提高,而HER降低,可以解释为FeS进行主要电子介导的TCE脱氯,而Fe氧化物进行次电子介导的HER。











































 京公网安备 11010802027423号
京公网安备 11010802027423号