当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Review of Hybrid Ion Capacitors: From Aqueous to Lithium to Sodium
Chemical Reviews ( IF 62.1 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.chemrev.8b00116 Jia Ding 1 , Wenbin Hu 2 , Eunsu Paek 3 , David Mitlin 3
Chemical Reviews ( IF 62.1 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.chemrev.8b00116 Jia Ding 1 , Wenbin Hu 2 , Eunsu Paek 3 , David Mitlin 3
Affiliation
|
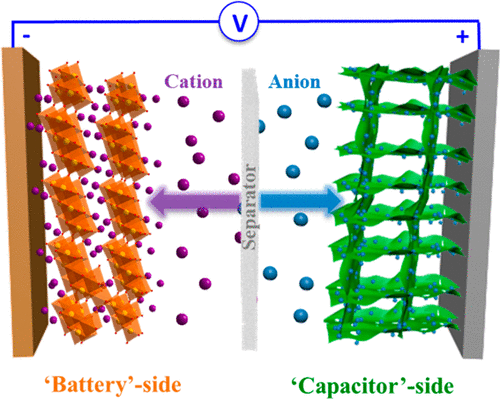
In this critical Review we focus on the evolution of the hybrid ion capacitor (HIC) from its early embodiments to its modern form, focusing on the key outstanding scientific and technological questions that necessitate further in-depth study. It may be argued that HICs began as aqueous systems, based on a Faradaic oxide positive electrode (e.g., Co3O4, RuOx) and an activated carbon ion-adsorption negative electrode. In these early embodiments HICs were meant to compete directly with electrical double layer capacitors (EDLCs), rather than with the much higher energy secondary batteries. The HIC design then evolved to be based on a wide voltage (∼4.2 V) carbonate-based battery electrolyte, using an insertion titanium oxide compound anode (Li4Ti5O12, LixTi5O12) versus a Li ion adsorption porous carbon cathode. The modern Na and Li architectures contain a diverse range of nanostructured materials in both electrodes, including TiO2, Li7Ti5O12, Li4Ti5O12, Na6LiTi5O12, Na2Ti3O7, graphene, hard carbon, soft carbon, graphite, carbon nanosheets, pseudocapacitor T-Nb2O5, V2O5, MXene, conversion compounds MoS2, VN, MnO, and Fe2O3/Fe3O4, cathodes based on Na3V2(PO4)3, NaTi2(PO4)3, sodium super ionic conductor (NASICON), etc. The Ragone chart characteristics of HIC devices critically depend on their anode–cathode architectures. Combining electrodes with the flattest capacity versus voltage characteristics, and the largest total voltage window, yields superior energy. Unfortunately “flat voltage” materials undergo significant volume expansion/contraction during cycling and are frequently lifetime limited. Overall more research on HIC cathodes is needed; apart from high surface area carbon, very few positive electrodes demonstrate the necessary 10 000 or 100 000 plus cycle life. It remains to be determined whether its lithium ion capacitors (LICs) or sodium ion capacitors (NICs) are superior in terms of energy–power and cyclability. We discuss unresolved issues, including poorly understood fast-charge storage mechanisms, prelithiation and presodiation, solid electrolyte interface (SEI) formation, and high-rate metal plating.
中文翻译:

混合离子电容器的综述:从水到锂再到钠
在这篇重要的综述中,我们着重于混合离子电容器(HIC)从其早期实施方案到现代形式的演变,着眼于需要进一步深入研究的关键性突出的科学和技术问题。可以认为,HICs是基于法拉第氧化正电极(例如Co 3 O 4,RuO x)和活性炭离子吸附负电极开始的水体系。在这些早期的实施例中,HIC旨在直接与双电层电容器(EDLC)竞争,而不是与能量更高的二次电池竞争。然后,HIC设计演变为基于宽电压(〜4.2 V)的碳酸盐基电池电解液,使用插入式二氧化钛化合物阳极(Li 4Ti 5 O 12,Li x Ti 5 O 12)相对于Li离子吸附多孔碳阴极。现代的Na和Li结构在两个电极中均包含多种纳米结构材料,包括TiO 2,Li 7 Ti 5 O 12,Li 4 Ti 5 O 12,Na 6 LiTi 5 O 12,Na 2 Ti 3 O 7,石墨烯,硬碳,软碳,石墨,碳纳米片,假电容器T -Nb 2 O5,V 2 O 5,MXene,转化化合物MoS 2,VN,MnO和Fe 2 O 3 / Fe 3 O 4,基于Na 3 V 2(PO 4)3,NaTi 2(PO 4)3的阴极,钠超离子导体(NASICON)等。HIC器件的Ragone图特性主要取决于其阳极-阴极体系结构。结合具有最平坦的容量与电压特性以及最大的总电压窗口的电极,可产生出众的能量。不幸的是,“平坦电压”材料在循环过程中会发生明显的体积膨胀/收缩,并且通常会限制使用寿命。总的来说,需要对HIC阴极进行更多的研究。除了高表面积碳外,极少的正极表现出必要的10 000或100 000加循环寿命。至于其锂离子电容器(LIC)或钠离子电容器(NIC)在能量,功率和可循环性方面是否优越,还有待确定。我们讨论了尚未解决的问题,包括人们对快速充电存储机制的了解不多,
更新日期:2018-06-28
中文翻译:

混合离子电容器的综述:从水到锂再到钠
在这篇重要的综述中,我们着重于混合离子电容器(HIC)从其早期实施方案到现代形式的演变,着眼于需要进一步深入研究的关键性突出的科学和技术问题。可以认为,HICs是基于法拉第氧化正电极(例如Co 3 O 4,RuO x)和活性炭离子吸附负电极开始的水体系。在这些早期的实施例中,HIC旨在直接与双电层电容器(EDLC)竞争,而不是与能量更高的二次电池竞争。然后,HIC设计演变为基于宽电压(〜4.2 V)的碳酸盐基电池电解液,使用插入式二氧化钛化合物阳极(Li 4Ti 5 O 12,Li x Ti 5 O 12)相对于Li离子吸附多孔碳阴极。现代的Na和Li结构在两个电极中均包含多种纳米结构材料,包括TiO 2,Li 7 Ti 5 O 12,Li 4 Ti 5 O 12,Na 6 LiTi 5 O 12,Na 2 Ti 3 O 7,石墨烯,硬碳,软碳,石墨,碳纳米片,假电容器T -Nb 2 O5,V 2 O 5,MXene,转化化合物MoS 2,VN,MnO和Fe 2 O 3 / Fe 3 O 4,基于Na 3 V 2(PO 4)3,NaTi 2(PO 4)3的阴极,钠超离子导体(NASICON)等。HIC器件的Ragone图特性主要取决于其阳极-阴极体系结构。结合具有最平坦的容量与电压特性以及最大的总电压窗口的电极,可产生出众的能量。不幸的是,“平坦电压”材料在循环过程中会发生明显的体积膨胀/收缩,并且通常会限制使用寿命。总的来说,需要对HIC阴极进行更多的研究。除了高表面积碳外,极少的正极表现出必要的10 000或100 000加循环寿命。至于其锂离子电容器(LIC)或钠离子电容器(NIC)在能量,功率和可循环性方面是否优越,还有待确定。我们讨论了尚未解决的问题,包括人们对快速充电存储机制的了解不多,



























 京公网安备 11010802027423号
京公网安备 11010802027423号