当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Substrate Recognition and Catalysis of the Haloalkanoic Acid Dehalogenase Family Member α-Phosphoglucomutase
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.biochem.8b00396 Chunchun Zhang 1 , Karen N Allen 2 , Debra Dunaway-Mariano 3
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.biochem.8b00396 Chunchun Zhang 1 , Karen N Allen 2 , Debra Dunaway-Mariano 3
Affiliation
|
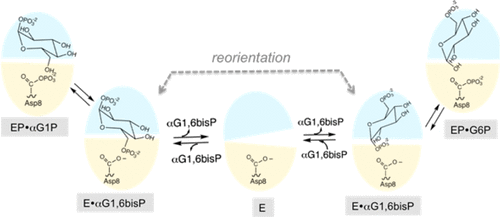
α-Phosphoglucomutase (αPGM), in its phosphorylated state, catalyzes the interconversion of α-d-glucose 1-phosphate and α-d-glucose 6-phosphate. The αPGM of Lactococcus lactis is a type C2B member of the haloalkanoic acid dehalogenase (HAD) enzyme family and is comprised of a Rossmann-fold catalytic domain and inserted α/β-fold cap domain. The active site is formed at the domain–domain interface. Herein, we report the results from a kinetic-based study of L. lactis αPGM catalysis, which demonstrate enzyme activation by autocatalyzed phosphorylation of Asp8 with αG1P, the intermediacy of αG1,6bisP in the phospho Ll-αPGM-catalyzed conversion of αG1P to G6P, and the reorientation of the αG1,6bisP intermediate via dissociation to solvent and rebinding. In order to provide insight into the structural determinants of L. lactis αPGM substrate recognition and catalysis, metal cofactor and substrate specificities were determined as were the contributions made by active-site residues toward catalytic efficiency. Lastly, the structure and catalytic mechanism of L. lactis αPGM are compared with those of HAD family phosphomutases L. lactis β-phosphoglucomutase and eukayotic α-phosphomannomutase to provide insight into the evolution of phosphohexomutases from HAD family phosphatases.
中文翻译:

卤代烷酸脱卤酶家族成员α-磷酸葡萄糖变位酶的底物识别和催化机制
α-磷酸葡萄糖变位酶 (αPGM) 在磷酸化状态下催化 α- d-葡萄糖 1-磷酸和 α- d-葡萄糖 6-磷酸的相互转化。乳酸乳球菌的 αPGM 是卤代烷酸脱卤酶 (HAD) 酶家族的 C2B 型成员,由罗斯曼折叠催化结构域和插入的 α/β 折叠帽结构域组成。活性位点形成于域-域界面处。在此,我们报告了基于动力学的乳酸乳球菌αPGM 催化研究的结果,该结果证明了通过 Asp8 与 αG1P 的自催化磷酸化来激活酶,αG1,6bisP 在磷酸化Ll -αPGM 催化的 αG1P 转化为 G6P 中起着中介作用,以及 αG1,6bisP 中间体通过解离到溶剂并重新结合而重新定向。为了深入了解乳酸乳球菌αPGM 底物识别和催化的结构决定因素,确定了金属辅因子和底物特异性以及活性位点残基对催化效率的贡献。最后,将乳酸乳球菌αPGM与HAD家族磷酸变位酶乳酸乳球菌β-磷酸葡萄糖变位酶和真核α-磷酸甘露糖变位酶的结构和催化机制进行比较,以深入了解HAD家族磷酸酶磷酸己糖变位酶的进化。
更新日期:2018-06-28
中文翻译:

卤代烷酸脱卤酶家族成员α-磷酸葡萄糖变位酶的底物识别和催化机制
α-磷酸葡萄糖变位酶 (αPGM) 在磷酸化状态下催化 α- d-葡萄糖 1-磷酸和 α- d-葡萄糖 6-磷酸的相互转化。乳酸乳球菌的 αPGM 是卤代烷酸脱卤酶 (HAD) 酶家族的 C2B 型成员,由罗斯曼折叠催化结构域和插入的 α/β 折叠帽结构域组成。活性位点形成于域-域界面处。在此,我们报告了基于动力学的乳酸乳球菌αPGM 催化研究的结果,该结果证明了通过 Asp8 与 αG1P 的自催化磷酸化来激活酶,αG1,6bisP 在磷酸化Ll -αPGM 催化的 αG1P 转化为 G6P 中起着中介作用,以及 αG1,6bisP 中间体通过解离到溶剂并重新结合而重新定向。为了深入了解乳酸乳球菌αPGM 底物识别和催化的结构决定因素,确定了金属辅因子和底物特异性以及活性位点残基对催化效率的贡献。最后,将乳酸乳球菌αPGM与HAD家族磷酸变位酶乳酸乳球菌β-磷酸葡萄糖变位酶和真核α-磷酸甘露糖变位酶的结构和催化机制进行比较,以深入了解HAD家族磷酸酶磷酸己糖变位酶的进化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号