当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
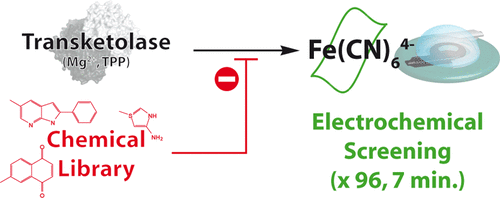
Innovative Electrochemical Screening Allows Transketolase Inhibitors to Be Identified
Analytical Chemistry ( IF 7.4 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.analchem.8b01752 Chloé M. G. Aymard 1 , Matilte Halma 2 , Arnaud Comte 1 , Christine Mousty 2 , Vanessa Prévot 2 , Laurence Hecquet 2 , Franck Charmantray 2 , Loïc J. Blum 1 , Bastien Doumèche 1
Analytical Chemistry ( IF 7.4 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.analchem.8b01752 Chloé M. G. Aymard 1 , Matilte Halma 2 , Arnaud Comte 1 , Christine Mousty 2 , Vanessa Prévot 2 , Laurence Hecquet 2 , Franck Charmantray 2 , Loïc J. Blum 1 , Bastien Doumèche 1
Affiliation
|
Transketolases (TKs) are ubiquitous thiamine pyrophosphate (TPP)-dependent enzymes of the nonoxidative branch of the pentose phosphate pathway. They are considered as interesting therapeutic targets in numerous diseases and infections (e.g., cancer, tuberculosis, malaria), for which it is important to find specific and efficient inhibitors. Current TK assays require important amounts of enzyme, are time-consuming, and are not specific. Here, we report a new high throughput electrochemical assay based on the oxidative trapping of the TK-TPP intermediate. After electrode characterization, the enzyme loading, electrochemical protocol, and substrate concentration were optimized. Finally, 96 electrochemical assays could be performed in parallel in only 7 min, which allows a rapid screening of TK inhibitors. Then, 1360 molecules of an in-house chemical library were screened and one early lead compound was identified to inhibit TK from E. coli with an IC50 of 63 μM and an inhibition constant (KI) of 3.4 μM. The electrochemical assay was also used to propose an inhibition mechanism.
中文翻译:

创新的电化学筛选技术可确定转酮醇酶抑制剂
转酮酶(TKs)是戊糖磷酸途径非氧化分支中普遍存在的硫胺素焦磷酸(TPP)依赖性酶。它们被认为是许多疾病和感染(例如,癌症,结核病,疟疾)中令人感兴趣的治疗靶标,为此,找到特异性和有效的抑制剂很重要。当前的TK测定需要大量的酶,既费时又不特异性。在这里,我们报告了基于TK-TPP中间体的氧化捕集的新的高通量电化学分析。电极表征后,对酶的负载,电化学方案和底物浓度进行了优化。最后,仅需7分钟即可并行执行96次电化学检测,从而可以快速筛选TK抑制剂。然后,大肠杆菌,IC 50为63μM,抑制常数(K I)为3.4μM。电化学测定也用于提出抑制机制。
更新日期:2018-06-28
中文翻译:

创新的电化学筛选技术可确定转酮醇酶抑制剂
转酮酶(TKs)是戊糖磷酸途径非氧化分支中普遍存在的硫胺素焦磷酸(TPP)依赖性酶。它们被认为是许多疾病和感染(例如,癌症,结核病,疟疾)中令人感兴趣的治疗靶标,为此,找到特异性和有效的抑制剂很重要。当前的TK测定需要大量的酶,既费时又不特异性。在这里,我们报告了基于TK-TPP中间体的氧化捕集的新的高通量电化学分析。电极表征后,对酶的负载,电化学方案和底物浓度进行了优化。最后,仅需7分钟即可并行执行96次电化学检测,从而可以快速筛选TK抑制剂。然后,大肠杆菌,IC 50为63μM,抑制常数(K I)为3.4μM。电化学测定也用于提出抑制机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号