当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Methane to Methanol: The Selectivity–Conversion Limit and Design Strategies
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1021/acscatal.8b00220 Allegra A. Latimer 1 , Arvin Kakekhani 1 , Ambarish R. Kulkarni 1 , Jens K. Nørskov 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1021/acscatal.8b00220 Allegra A. Latimer 1 , Arvin Kakekhani 1 , Ambarish R. Kulkarni 1 , Jens K. Nørskov 1, 2
Affiliation
|
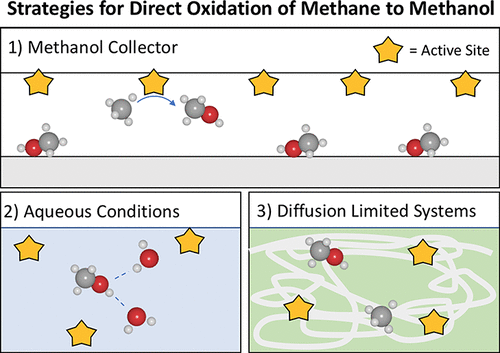
Currently, methane is transformed into methanol through the two-step syngas process, which requires high temperatures and centralized production. While the slightly exothermic direct partial oxidation of methane to methanol would be preferable, no such process has been established despite over a century of research. Generally, this failure has been attributed to both the high barriers required to activate methane as well as the higher activity of the CH bonds in methanol compared to those in methane. However, a precise and general quantification of the limitations of catalytic direct methane to methanol has yet to be established. Herein, we present a simple kinetic model to explain the selectivity–conversion trade-off that hampers continuous partial oxidation of methane to methanol. For the same kinetic model, we apply two distinct methods, (1) using ab initio calculations and (2) fitting to a large experimental database, to fully define the model parameters. We find that both methods yield strikingly similar results, namely, that the selectivity of methane to methanol in a direct, continuous process can be fully described by the methane conversion, the temperature, and a catalyst-independent difference in methane and methanol activation free energies, ΔGa, which is dictated by the relative reactivity of the C–H bonds in methane and methanol. Stemming from this analysis, we suggest several design strategies for increasing methanol yields under the constraint of constant ΔGa. These strategies include (1) “collectors”, materials with strong methanol adsorption potential that can help to lower the partial pressure of methanol in the gas phase, (2) aqueous reaction conditions, and/or (3) diffusion-limited systems. By using this simple model to successfully rationalize a representative library of experimental studies from the diverse fields of heterogeneous, homogeneous, biological, and gas-phase methane to methanol catalysis, we underscore the idea that continuous methane to methanol is generally limited and provide a framework for understanding and evaluating new catalysts and processes.
中文翻译:

直接甲烷制甲醇:选择性-转化极限和设计策略
当前,甲烷通过两步合成气工艺转化为甲醇,这需要高温和集中生产。尽管将甲烷稍微放热的直接部分氧化为甲醇是优选的,但是尽管经过了一个多世纪的研究,仍没有建立这种方法。通常,该失败归因于活化甲烷所需的高壁垒以及与甲烷中的CH键相比,甲醇中的CH键具有更高的活性。然而,尚未建立对催化直接甲烷转化为甲醇的局限性的精确和一般的量化。在这里,我们提供了一个简单的动力学模型来解释选择性-转化的折衷方案,这阻碍了甲烷连续部分氧化为甲醇。对于相同的动力学模型,我们采用两种不同的方法,(1)使用从头算式和(2)拟合大型实验数据库,以完全定义模型参数。我们发现这两种方法均产生惊人相似的结果,即在直接,连续过程中甲烷对甲醇的选择性可以通过甲烷转化率,温度以及甲烷和甲醇活化自由能的催化剂独立差异来充分描述。 ,ΔG a,由甲烷和甲醇中C–H键的相对反应性决定。从这一分析中得出结论,我们提出了在恒定ΔG a约束下提高甲醇收率的几种设计策略。。这些策略包括(1)“收集器”,具有很强的甲醇吸附潜能的材料,可以帮助降低气相中甲醇的分压,(2)水性反应条件和/或(3)扩散受限的系统。通过使用这个简单的模型成功地合理化了从异质,均相,生物和气相甲烷到甲醇催化的不同领域的实验研究的代表性库,我们强调了甲烷到甲醇的连续甲烷通常受到限制的想法,并提供了一个框架用于了解和评估新的催化剂和工艺。
更新日期:2018-06-29
中文翻译:

直接甲烷制甲醇:选择性-转化极限和设计策略
当前,甲烷通过两步合成气工艺转化为甲醇,这需要高温和集中生产。尽管将甲烷稍微放热的直接部分氧化为甲醇是优选的,但是尽管经过了一个多世纪的研究,仍没有建立这种方法。通常,该失败归因于活化甲烷所需的高壁垒以及与甲烷中的CH键相比,甲醇中的CH键具有更高的活性。然而,尚未建立对催化直接甲烷转化为甲醇的局限性的精确和一般的量化。在这里,我们提供了一个简单的动力学模型来解释选择性-转化的折衷方案,这阻碍了甲烷连续部分氧化为甲醇。对于相同的动力学模型,我们采用两种不同的方法,(1)使用从头算式和(2)拟合大型实验数据库,以完全定义模型参数。我们发现这两种方法均产生惊人相似的结果,即在直接,连续过程中甲烷对甲醇的选择性可以通过甲烷转化率,温度以及甲烷和甲醇活化自由能的催化剂独立差异来充分描述。 ,ΔG a,由甲烷和甲醇中C–H键的相对反应性决定。从这一分析中得出结论,我们提出了在恒定ΔG a约束下提高甲醇收率的几种设计策略。。这些策略包括(1)“收集器”,具有很强的甲醇吸附潜能的材料,可以帮助降低气相中甲醇的分压,(2)水性反应条件和/或(3)扩散受限的系统。通过使用这个简单的模型成功地合理化了从异质,均相,生物和气相甲烷到甲醇催化的不同领域的实验研究的代表性库,我们强调了甲烷到甲醇的连续甲烷通常受到限制的想法,并提供了一个框架用于了解和评估新的催化剂和工艺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号