当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron Carbidization on Thin-Film Silica and Silicon: A Near-Ambient-Pressure X-ray Photoelectron Spectroscopy and Scanning Tunneling Microscopy Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acscatal.8b02076 Xiong Zhou 1, 2 , Gilbère J. A. Mannie 1 , Junqing Yin 1 , Xin Yu 1, 2 , C. J. Weststrate 3 , Xiaodong Wen 1, 2 , Kai Wu 4 , Yong Yang 1, 2 , Yongwang Li 1, 2 , J. W. Niemantsverdriet 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acscatal.8b02076 Xiong Zhou 1, 2 , Gilbère J. A. Mannie 1 , Junqing Yin 1 , Xin Yu 1, 2 , C. J. Weststrate 3 , Xiaodong Wen 1, 2 , Kai Wu 4 , Yong Yang 1, 2 , Yongwang Li 1, 2 , J. W. Niemantsverdriet 1, 3
Affiliation
|
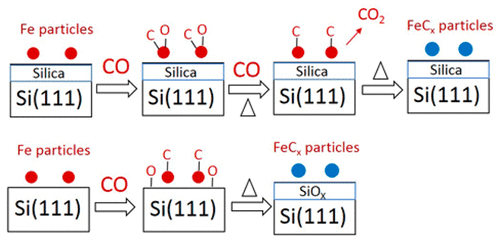
Model catalysts consisting of iron particles with similar size deposited on thin-film silica (Fe/SiO2) and on silicon (Fe/Si) were used to study iron carbidization in a CO atmosphere using in situ near-ambient-pressure X-ray photoelectron spectroscopy. Significant differences were observed for CO adsorption, CO dissociation, and iron carbidization when the support was changed from thin-film silica to silicon. Stronger adsorption of CO on Fe/Si than that on Fe/SiO2 was evident from the higher CO equilibrium coverage found at a given temperature in the presence of 1 mbar of CO gas. On thin-film silica, iron starts to carbidize at 150 °C, while the onset of carbidization is at 100 °C on the silicon support. The main reason for the different onset temperature for carbidization is the efficiency of removal of oxygen species after CO dissociation. On thin-film silica, oxygen species formed by CO dissociation block the iron surface until ∼150 °C, when CO2 formation removes surface oxygen. Instead, on the silicon support, oxygen species readily spill over to the silicon. As a consequence, oxygen removal is not rate-limiting anymore and carbidization of iron can proceed at a lower temperature.
中文翻译:

薄膜二氧化硅和硅上的铁碳化:近环境压力X射线光电子能谱和扫描隧道显微镜研究
模型催化剂由沉积在薄膜二氧化硅(Fe / SiO 2)和硅(Fe / Si)上的具有相似尺寸的铁颗粒组成,用于通过原位近环境X射线研究CO气氛中的铁碳化作用光电子能谱。当载体从薄膜二氧化硅变为硅时,观察到CO吸附,CO解离和铁碳化的显着差异。与Fe / SiO 2相比,CO在Fe / Si上的吸附更强从在1 mbar CO气体存在下在给定温度下发现的较高的CO平衡覆盖率可以明显看出这一点。在薄膜二氧化硅上,铁在150°C时开始发生碳化,而硅载体上的碳化开始于100°C。碳化起始温度不同的主要原因是一氧化碳解离后去除氧的效率高。在薄膜二氧化硅上,由CO分解形成的氧会阻塞铁表面,直到〜150°C,此时CO 2的形成会去除表面的氧。相反,在硅载体上,氧很容易溢出到硅上。结果,除氧不再是限速的,铁的碳化可以在较低的温度下进行。
更新日期:2018-06-28
中文翻译:

薄膜二氧化硅和硅上的铁碳化:近环境压力X射线光电子能谱和扫描隧道显微镜研究
模型催化剂由沉积在薄膜二氧化硅(Fe / SiO 2)和硅(Fe / Si)上的具有相似尺寸的铁颗粒组成,用于通过原位近环境X射线研究CO气氛中的铁碳化作用光电子能谱。当载体从薄膜二氧化硅变为硅时,观察到CO吸附,CO解离和铁碳化的显着差异。与Fe / SiO 2相比,CO在Fe / Si上的吸附更强从在1 mbar CO气体存在下在给定温度下发现的较高的CO平衡覆盖率可以明显看出这一点。在薄膜二氧化硅上,铁在150°C时开始发生碳化,而硅载体上的碳化开始于100°C。碳化起始温度不同的主要原因是一氧化碳解离后去除氧的效率高。在薄膜二氧化硅上,由CO分解形成的氧会阻塞铁表面,直到〜150°C,此时CO 2的形成会去除表面的氧。相反,在硅载体上,氧很容易溢出到硅上。结果,除氧不再是限速的,铁的碳化可以在较低的温度下进行。







































 京公网安备 11010802027423号
京公网安备 11010802027423号