当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
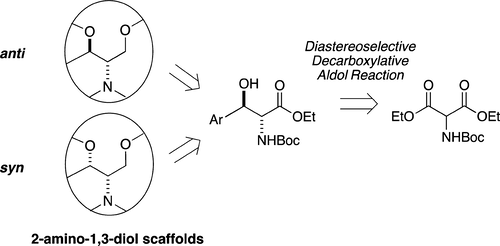
Access to Anti or Syn 2-Amino-1,3-diol Scaffolds from a Common Decarboxylative Aldol Adduct
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.joc.8b00901 Pauline Chaumont 1 , Jérome Baudoux 2 , Jacques Maddaluno 1 , Jacques Rouden 2 , Anne Harrison-Marchand 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.joc.8b00901 Pauline Chaumont 1 , Jérome Baudoux 2 , Jacques Maddaluno 1 , Jacques Rouden 2 , Anne Harrison-Marchand 1
Affiliation
|
A straightforward synthetic pathway allowing the access to anti or syn 2-amino-1,3-diol scaffolds is presented. The strategy relies on a diastereoselective organocatalyzed decarboxylative aldol reaction of a N-Boc-hemimalonate that is easily formed from commercial N-Boc-diethyl malonate. Although this method has been optimized previously with the N-Bz-hemimalonate analogue, this key step was reinvestigated with the N-Boc derivative to improve the required reaction time, the yield, and the diastereoselectivity. The new conditions enhance this transformation, and quantitative yields and anti/syn ratios up to 96:4 can be obtained. The anti aldol product was easily isolated in pure form and then taken forward as the key precursor in the preparation of both a set of ten N-/O-alkylated anti 2-amino-1,3-diol derivatives and the syn congeners.
中文翻译:

从常见的脱羧醛醇加合物获得抗或Syn 2-氨基-1,3-二醇支架
提出了一种直接的合成途径,其允许接近抗或同位的2-氨基-1,3-二醇支架。该策略依赖于N -Boc-半棉酸酯的非对映选择性有机催化的脱羧醛醇缩合反应,其易于由商业化的N -Boc-二乙基丙二酸酯形成。尽管此方法之前已使用N -Bz-hemimalonate类似物进行了优化,但这一关键步骤已由N重新进行了研究。-Boc衍生物可改善所需的反应时间,收率和非对映选择性。新条件增强了这种转化,并且可以获得高达96:4的定量产率和反/合成比。可以容易地以纯净形式分离出抗羟醛产物,然后将其作为制备十种N- / O-烷基化的抗2-氨基-1,3-二醇衍生物和同系同源物的关键前体。
更新日期:2018-06-28
中文翻译:

从常见的脱羧醛醇加合物获得抗或Syn 2-氨基-1,3-二醇支架
提出了一种直接的合成途径,其允许接近抗或同位的2-氨基-1,3-二醇支架。该策略依赖于N -Boc-半棉酸酯的非对映选择性有机催化的脱羧醛醇缩合反应,其易于由商业化的N -Boc-二乙基丙二酸酯形成。尽管此方法之前已使用N -Bz-hemimalonate类似物进行了优化,但这一关键步骤已由N重新进行了研究。-Boc衍生物可改善所需的反应时间,收率和非对映选择性。新条件增强了这种转化,并且可以获得高达96:4的定量产率和反/合成比。可以容易地以纯净形式分离出抗羟醛产物,然后将其作为制备十种N- / O-烷基化的抗2-氨基-1,3-二醇衍生物和同系同源物的关键前体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号