当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Approach to Chiral 1-Substituted Isoquinolone and 3-Substituted Isoindolin-1-one by Addition–Cyclization Process
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.joc.8b01282 Wen Zhou 1 , Yan-Xue Zhang 1 , Xiao-Di Nie 1 , Chang-Mei Si 1 , Xun Sun 1 , Bang-Guo Wei 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.joc.8b01282 Wen Zhou 1 , Yan-Xue Zhang 1 , Xiao-Di Nie 1 , Chang-Mei Si 1 , Xun Sun 1 , Bang-Guo Wei 1
Affiliation
|
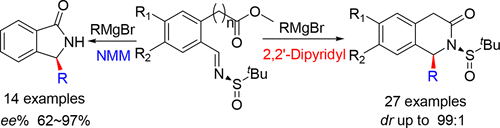
An approach to access 1-substituted isoquinolones has been developed through the addition–cyclization of imines with Grignard reagents in the presence of 2,2′-dipyridyl. A number of substituted aromatic magnesium reagents were amenable to this process, and the desired products were obtained with excellent yields and outstanding diastereoselectivities (dr > 99:1). The utility of this convenient approach is demonstrated by the formal synthesis of (S)-cryptostyline II. Moreover, N-methylmorpholine (NMM) was found to be an effective additive for the formation of 3-substituted isoindolin-1-ones using one-pot addition–cyclization–deprotection of imine with Grignard reagents.
中文翻译:

加成环化法制备手性1-取代异喹诺酮和3-取代异吲哚啉-1-酮
通过在2,2'-联吡啶存在下,用格氏试剂对亚胺进行加成环化反应,已经开发了一种获得1-取代异喹诺酮的方法。许多取代的芳族镁试剂都适用于此过程,并以优异的收率和出色的非对映选择性(dr > 99:1)获得了所需的产物。这种便利方法的实用性通过(S)-cryptostyline II的形式合成得到证明。此外,使用格氏试剂一锅加成-环化-亚胺脱保护,发现N-甲基吗啉(NMM)是形成3取代异吲哚啉-1-酮的有效添加剂。
更新日期:2018-06-28
中文翻译:

加成环化法制备手性1-取代异喹诺酮和3-取代异吲哚啉-1-酮
通过在2,2'-联吡啶存在下,用格氏试剂对亚胺进行加成环化反应,已经开发了一种获得1-取代异喹诺酮的方法。许多取代的芳族镁试剂都适用于此过程,并以优异的收率和出色的非对映选择性(dr > 99:1)获得了所需的产物。这种便利方法的实用性通过(S)-cryptostyline II的形式合成得到证明。此外,使用格氏试剂一锅加成-环化-亚胺脱保护,发现N-甲基吗啉(NMM)是形成3取代异吲哚啉-1-酮的有效添加剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号