当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Regioselective Hydroaminocarbonylation of Alkynes to α,β-Unsaturated Primary Amides with Ammonium Chloride
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.joc.8b01405 Xiaolei Ji 1 , Bao Gao 2 , Xibing Zhou 2 , Zongjian Liu 1 , Hanmin Huang 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1021/acs.joc.8b01405 Xiaolei Ji 1 , Bao Gao 2 , Xibing Zhou 2 , Zongjian Liu 1 , Hanmin Huang 1, 2
Affiliation
|
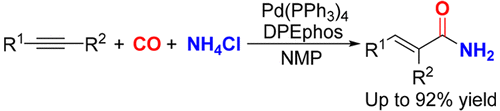
α,β-Unsaturated primary amides have found numerous applications in drug development, organic materials, and polymer sciences. However, the catalytic synthesis of α,β-unsaturated primary amides via carbonylation of alkynes has long been an elusive endeavor. Here, we report a novel palladium-catalyzed hydroaminocarbonylation of alkynes with NH4Cl as the amine source, enabling the highly chemo- and regioselective synthesis of α,β-unsaturated primary amides. A variety of alkynes, including aromatic alkynes, aliphatic alkynes, terminal alkynes, internal alkynes, as well as diynes with various functional groups, react well. The method turns the parasitic noncoordination ability of ammonium salts into a strategic advantage, enabling the gram-scale reaction to be performed in the presence of 0.05 mol % of catalyst with excellent selectivity
中文翻译:

钯催化炔烃对α,β-不饱和伯酰胺的炔烃区域选择性氢氨基羰基化反应
α,β-不饱和伯酰胺在药物开发,有机材料和聚合物科学中发现了许多应用。然而,长期以来,通过炔烃的羰基化催化合成α,β-不饱和伯酰胺一直是一项艰巨的努力。在这里,我们报告一种新型的钯催化炔烃与NH 4的加氢氨基羰基化反应Cl作为胺源,可以高度化学和区域选择性地合成α,β-不饱和伯酰胺。包括芳族炔烃,脂族炔烃,末端炔烃,内部炔烃以及具有各种官能团的二炔在内的多种炔烃反应良好。该方法将铵盐的寄生非配位能力转变为战略优势,从而使克级反应可以在0.05 mol%的催化剂存在下进行,并且具有出色的选择性。
更新日期:2018-06-28
中文翻译:

钯催化炔烃对α,β-不饱和伯酰胺的炔烃区域选择性氢氨基羰基化反应
α,β-不饱和伯酰胺在药物开发,有机材料和聚合物科学中发现了许多应用。然而,长期以来,通过炔烃的羰基化催化合成α,β-不饱和伯酰胺一直是一项艰巨的努力。在这里,我们报告一种新型的钯催化炔烃与NH 4的加氢氨基羰基化反应Cl作为胺源,可以高度化学和区域选择性地合成α,β-不饱和伯酰胺。包括芳族炔烃,脂族炔烃,末端炔烃,内部炔烃以及具有各种官能团的二炔在内的多种炔烃反应良好。该方法将铵盐的寄生非配位能力转变为战略优势,从而使克级反应可以在0.05 mol%的催化剂存在下进行,并且具有出色的选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号