当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular Hydrogen Bonding Enhances Stability and Reactivity of Mononuclear Cupric Superoxide Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-29 , DOI: 10.1021/jacs.8b04671 Mayukh Bhadra 1 , Jung Yoon C Lee 1 , Ryan E Cowley 2 , Sunghee Kim 1 , Maxime A Siegler 1 , Edward I Solomon 2 , Kenneth D Karlin 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-29 , DOI: 10.1021/jacs.8b04671 Mayukh Bhadra 1 , Jung Yoon C Lee 1 , Ryan E Cowley 2 , Sunghee Kim 1 , Maxime A Siegler 1 , Edward I Solomon 2 , Kenneth D Karlin 1
Affiliation
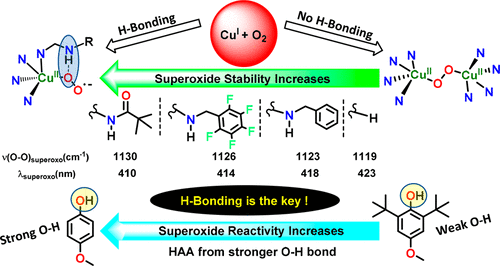
|
[(L)CuII(O2•-)]+ (i.e., cupric-superoxo) complexes, as the first and/or key reactive intermediates in (bio)chemical Cu-oxidative processes, including in the monooxygenases PHM and DβM, have been systematically stabilized by intramolecular hydrogen bonding within a TMPA ligand-based framework. Also, gradual strengthening of ligand-derived H-bonding dramatically enhances the [(L)CuII(O2•-)]+ reactivity toward hydrogen-atom abstraction (HAA) of phenolic O-H bonds. Spectroscopic properties of the superoxo complexes and their azido analogues, [(L)CuII(N3-)]+, also systematically change as a function of ligand H-bonding capability.
中文翻译:

分子内氢键增强单核超氧化物铜配合物的稳定性和反应性
[(L)CuII(O2•-)]+(即铜-超氧)配合物,作为(生物)化学铜氧化过程(包括单加氧酶 PHM 和 DβM)中的第一个和/或关键反应中间体,已被通过基于 TMPA 配体的框架内的分子内氢键系统地稳定。此外,配体衍生的氢键的逐渐增强显着增强了 [(L)CuII(O2•-)]+ 对酚 OH 键的氢原子夺取 (HAA) 的反应性。超氧配合物及其叠氮类似物 [(L)CuII(N3-)]+ 的光谱特性也会随着配体氢键能力的变化而系统地变化。
更新日期:2018-06-29
中文翻译:

分子内氢键增强单核超氧化物铜配合物的稳定性和反应性
[(L)CuII(O2•-)]+(即铜-超氧)配合物,作为(生物)化学铜氧化过程(包括单加氧酶 PHM 和 DβM)中的第一个和/或关键反应中间体,已被通过基于 TMPA 配体的框架内的分子内氢键系统地稳定。此外,配体衍生的氢键的逐渐增强显着增强了 [(L)CuII(O2•-)]+ 对酚 OH 键的氢原子夺取 (HAA) 的反应性。超氧配合物及其叠氮类似物 [(L)CuII(N3-)]+ 的光谱特性也会随着配体氢键能力的变化而系统地变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号