当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coordinatively Unsaturated [RhCp*Rf2] (Cp* = C5Me5; Rf = C6F3Cl2-3,5), General Precursor to Cp*-Diaryl and Cp*-Halo-Aryl RhIII Complexes. Observing and Testing the Effect of Cp* as Electronic Buffer
Organometallics ( IF 2.5 ) Pub Date : 2018-06-28 , DOI: 10.1021/acs.organomet.8b00227 Marconi N. Peñas-Defrutos 1 , Camino Bartolomé 1 , Pablo Espinet 1
Organometallics ( IF 2.5 ) Pub Date : 2018-06-28 , DOI: 10.1021/acs.organomet.8b00227 Marconi N. Peñas-Defrutos 1 , Camino Bartolomé 1 , Pablo Espinet 1
Affiliation
|
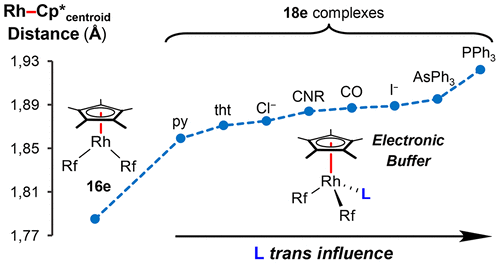
The pentacoordinated [RhCp*Rf2] (Rf = C6F3Cl2-3,5) and the octahedral (μ-Cl)2[RhCp*Rf]2, obtained by stoichiometric rearrangement with (μ-Cl)2[RhCp*Cl]2, are general precursors of [RhCp*RfXL] (X = Rf, Cl; L = ligand) complexes, which were studied by NMR (L dissociation and fluxional processes) and X-ray diffraction (structural effects affecting the Rh–Cp* distances) techniques. The Rh–Cp*centroid distances decrease markedly for identical L in the order [RhCp*Rf2L] > [RhCp*RfClL] > [RhCp*Cl2L] and are further influenced regularly within each family by the trans influence of L (longer distances for higher trans influence of L). The structural effects observed reveal a remarkable capability of Cp* to act as an electron-density buffer, which attenuates the Rh electron density variations induced by the substituents in front of Cp* by releasing toward Rh or polarizing toward Cp*, on demand, the electron density of the Rh–Cp* bonds. This buffer effect explains the easy L dissociation from [RhCp*Rf2L] and the accessibility to formally 16e pentacoordinated [RhCp*Rf2].
中文翻译:

配位不饱和的[RhCp * Rf 2 ](Cp * = C 5 Me 5; Rf = C 6 F 3 Cl 2 -3,5),Cp *-二芳基和Cp *-卤代芳基Rh III配合物的一般前体。观察和测试Cp *作为电子缓冲液的效果
五配位[RhCp * Rf 2 ](Rf = C 6 F 3 Cl 2 -3,5)和八面体(μ-Cl)2 [RhCp * Rf] 2,是通过(μ-Cl)2 [ ]化学计量重排获得的RhCp * Cl] 2是[RhCp * RfXL](X = Rf,Cl; L =配体)配合物的一般前体,已通过NMR(L解离和通量过程)和X射线衍射(影响结构的结构效应)进行了研究。 Rh–Cp *距离)技术。对于相同的L,Rh–Cp *质心距离以[RhCp * Rf 2 L]> [RhCp * RfClL]> [RhCp * Cl 2 L]的顺序显着减小,并且在每个家族中受到L的反式影响(距离越长,L的反式影响越大)。观察到的结构效应揭示了Cp *充当电子密度缓冲剂的显着能力,该能力可通过向Rh释放或朝Cp *极化来减弱Cp *前面的取代基引起的Rh电子密度变化。 Rh–Cp *键的电子密度。这种缓冲作用说明了容易从[RhCp * Rf 2 L]分离出L,以及可以容易地获得正式配位的16e五配位[RhCp * Rf 2 ]。
更新日期:2018-06-30
中文翻译:

配位不饱和的[RhCp * Rf 2 ](Cp * = C 5 Me 5; Rf = C 6 F 3 Cl 2 -3,5),Cp *-二芳基和Cp *-卤代芳基Rh III配合物的一般前体。观察和测试Cp *作为电子缓冲液的效果
五配位[RhCp * Rf 2 ](Rf = C 6 F 3 Cl 2 -3,5)和八面体(μ-Cl)2 [RhCp * Rf] 2,是通过(μ-Cl)2 [ ]化学计量重排获得的RhCp * Cl] 2是[RhCp * RfXL](X = Rf,Cl; L =配体)配合物的一般前体,已通过NMR(L解离和通量过程)和X射线衍射(影响结构的结构效应)进行了研究。 Rh–Cp *距离)技术。对于相同的L,Rh–Cp *质心距离以[RhCp * Rf 2 L]> [RhCp * RfClL]> [RhCp * Cl 2 L]的顺序显着减小,并且在每个家族中受到L的反式影响(距离越长,L的反式影响越大)。观察到的结构效应揭示了Cp *充当电子密度缓冲剂的显着能力,该能力可通过向Rh释放或朝Cp *极化来减弱Cp *前面的取代基引起的Rh电子密度变化。 Rh–Cp *键的电子密度。这种缓冲作用说明了容易从[RhCp * Rf 2 L]分离出L,以及可以容易地获得正式配位的16e五配位[RhCp * Rf 2 ]。











































 京公网安备 11010802027423号
京公网安备 11010802027423号