当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon Dioxide Reduction by Iron Hangman Porphyrins
Organometallics ( IF 2.5 ) Pub Date : 2018-06-28 , DOI: 10.1021/acs.organomet.8b00334 Charles G. Margarit 1 , Christoph Schnedermann 1 , Naomi G. Asimow 1 , Daniel G. Nocera 1
Organometallics ( IF 2.5 ) Pub Date : 2018-06-28 , DOI: 10.1021/acs.organomet.8b00334 Charles G. Margarit 1 , Christoph Schnedermann 1 , Naomi G. Asimow 1 , Daniel G. Nocera 1
Affiliation
|
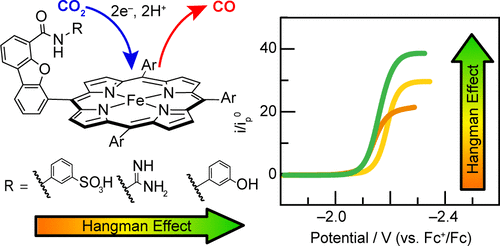
Iron hangman porphyrins with phenol, guanidinium, and sulfonic acid proton donor groups placed above the Fe porphyrin platform reduce CO2 to CO with Faradaic efficiencies >93%. Computations show that the activation of CO2 at the Fe center is enhanced by the hanging group. Intramolecular hydrogen bonding from the phenol and guanidinium groups results in a 2.1–6.6 kcal/mol stabilization of CO2 within the hangman pocket; the hanging sulfonate group is deprotonated, thus resulting in destabilization of the CO2 adduct due to unfavorable electrostatic interactions. Electrochemical studies show that Fe hangman porphyrins exhibit canonical S-curve character; together with computation results, the apparent rate constant for CO2 reduction is found to be governed by CO2 binding within the hangman cleft.
中文翻译:

铁Hang子卟啉可减少二氧化碳排放
置于Fe卟啉平台上方的具有苯酚,胍盐和磺酸质子供体基团的Hangman卟啉铁可将法拉第效率> 93%的CO 2转化为CO。计算表明,悬挂基团增强了Fe中心的CO 2活化。苯酚和胍基团之间的分子内氢键使子手口袋内的CO 2稳定在2.1–6.6 kcal / mol 。悬挂的磺酸根基团被去质子化,由于不利的静电相互作用而导致CO 2加合物的不稳定。电化学研究表明,Fe hangman卟啉具有典型的S曲线特征。连同计算结果,CO 2的表观速率常数发现还原是由子手裂隙内的CO 2结合决定的。
更新日期:2019-02-06
中文翻译:

铁Hang子卟啉可减少二氧化碳排放
置于Fe卟啉平台上方的具有苯酚,胍盐和磺酸质子供体基团的Hangman卟啉铁可将法拉第效率> 93%的CO 2转化为CO。计算表明,悬挂基团增强了Fe中心的CO 2活化。苯酚和胍基团之间的分子内氢键使子手口袋内的CO 2稳定在2.1–6.6 kcal / mol 。悬挂的磺酸根基团被去质子化,由于不利的静电相互作用而导致CO 2加合物的不稳定。电化学研究表明,Fe hangman卟啉具有典型的S曲线特征。连同计算结果,CO 2的表观速率常数发现还原是由子手裂隙内的CO 2结合决定的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号