当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Improved Delivery to Breast Cancer Based on a Novel Nanocarrier Modified with High‐Affinity Peptides Discovered by Phage Display
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-06-28 , DOI: 10.1002/adhm.201800269 Haoran Zhang 1 , Zhaoming Guo 2 , Bing He 1 , Wenbing Dai 1 , Hua Zhang 1 , Xueqing Wang 1 , Qiang Zhang 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2018-06-28 , DOI: 10.1002/adhm.201800269 Haoran Zhang 1 , Zhaoming Guo 2 , Bing He 1 , Wenbing Dai 1 , Hua Zhang 1 , Xueqing Wang 1 , Qiang Zhang 1
Affiliation
|
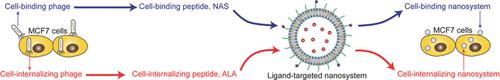
Ligand‐targeted nanosystems have the potential to realize site‐specific tumor therapy and alleviate unwanted side effects of many chemotherapeutic agents, and one of the most key issues seems to be the construction of an effective nanocarrier. Based on different processes of phage display techniques, 38 cell‐binding peptides and 32 cell‐internalizing peptides are discovered. Four of these ligand peptides [FIPFDPMSMRWE (FIP), NASSFPTNSRWA (NAS), GLHTSATNLYLH (GLH), and ALAVAPSRWWNE (ALA), respectively] exhibit high affinity to MCF7 human breast cancer cells. Among them, NAS and ALA are reported for the first time, whose affinities are 20.6 and 76.3 times that of the random peptide control, respectively. Both NAS and ALA modifications to doxorubicin‐loaded lipid nanosytems [LP(DOX)] show stronger tumor inhibition, longer animal survival time, and less body weight loss, compared to unmodified or control peptide modified nanosystems, on an MCF7 tumor‐bearing mouse model. In conclusion, the cell‐binding peptide NAS and cell‐internalizing peptide ALA can be used for ligand‐targeted delivery of antitumor drugs. It seems that the in vivo antitumor effect of these ligand‐targeted nanosystems is closely related to their ligand–cell affinity, but fairly tolerant of the ligand types.
中文翻译:

基于通过噬菌体展示发现的高亲和力肽修饰的新型纳米载体,提高了乳腺癌的分娩率
靶向配体的纳米系统有潜力实现针对特定部位的肿瘤治疗并减轻许多化疗药物的不良副作用,而最关键的问题之一似乎是构建有效的纳米载体。根据噬菌体展示技术的不同过程,发现了38种细胞结合肽和32种细胞内在化肽。这些配体肽中的四个[FIPFDPMSMRWE(FIP),NASSFPTNSRWA(NAS),GLHTSATNLYLH(GLH)和ALAVAPSRWWNE(ALA)分别对MCF7人乳腺癌细胞表现出高亲和力。其中,首次报道了NAS和ALA,其亲和力分别是随机肽对照的20.6和76.3倍。NAS和ALA对载有阿霉素的脂质纳米系统[LP(DOX)]的修饰均显示出更强的肿瘤抑制作用,更长的动物存活时间,与未修饰或对照肽修饰的纳米系统相比,在带有MCF7荷瘤小鼠模型上,体重减轻的幅度较小。总之,细胞结合肽NAS和细胞内在化肽ALA可用于抗肿瘤药物的配体靶向递送。这些以配体为靶标的纳米系统的体内抗肿瘤作用似乎与其配体-细胞亲和力密切相关,但对配体类型的耐受性却很高。
更新日期:2018-06-28
中文翻译:

基于通过噬菌体展示发现的高亲和力肽修饰的新型纳米载体,提高了乳腺癌的分娩率
靶向配体的纳米系统有潜力实现针对特定部位的肿瘤治疗并减轻许多化疗药物的不良副作用,而最关键的问题之一似乎是构建有效的纳米载体。根据噬菌体展示技术的不同过程,发现了38种细胞结合肽和32种细胞内在化肽。这些配体肽中的四个[FIPFDPMSMRWE(FIP),NASSFPTNSRWA(NAS),GLHTSATNLYLH(GLH)和ALAVAPSRWWNE(ALA)分别对MCF7人乳腺癌细胞表现出高亲和力。其中,首次报道了NAS和ALA,其亲和力分别是随机肽对照的20.6和76.3倍。NAS和ALA对载有阿霉素的脂质纳米系统[LP(DOX)]的修饰均显示出更强的肿瘤抑制作用,更长的动物存活时间,与未修饰或对照肽修饰的纳米系统相比,在带有MCF7荷瘤小鼠模型上,体重减轻的幅度较小。总之,细胞结合肽NAS和细胞内在化肽ALA可用于抗肿瘤药物的配体靶向递送。这些以配体为靶标的纳米系统的体内抗肿瘤作用似乎与其配体-细胞亲和力密切相关,但对配体类型的耐受性却很高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号