当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oligomer Diversity during the Aggregation of the Repeat Region of Tau.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-07-17 , DOI: 10.1021/acschemneuro.8b00250 Magnus Kjaergaard 1, 2 , Alexander J Dear 1 , Franziska Kundel 1 , Seema Qamar 3 , Georg Meisl 1 , Tuomas P J Knowles 1, 4 , David Klenerman 1, 5
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-07-17 , DOI: 10.1021/acschemneuro.8b00250 Magnus Kjaergaard 1, 2 , Alexander J Dear 1 , Franziska Kundel 1 , Seema Qamar 3 , Georg Meisl 1 , Tuomas P J Knowles 1, 4 , David Klenerman 1, 5
Affiliation
|
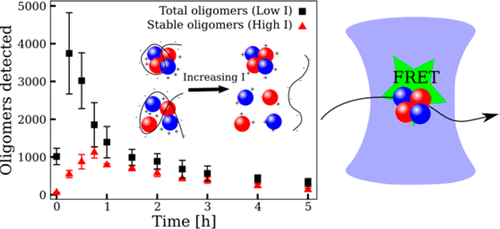
The molecular mechanism of protein aggregation is of both fundamental and clinical importance as amyloid aggregates are linked to a number of neurodegenerative disorders. Such protein aggregates include macroscopic insoluble fibrils as well as small soluble oligomeric species. Time-dependent resolution of these species is prerequisite for a detailed quantitative understanding of protein aggregation; this remains challenging due to the lack of methods for detecting and characterizing transient and heterogeneous protein oligomers. Here we have used single molecule fluorescence techniques combined with mechanistic modeling to study the heparin-induced aggregation of the repeat region of tau, which forms the core region of neurofibrillary tangles found in Alzheimer's disease. We distinguish several subpopulations of oligomers with different stability and follow their evolution during aggregation reactions as a function of temperature and concentration. Employment of techniques from chemical kinetics reveals that the two largest populations are structurally distinct from fibrils and are both kinetically and thermodynamically unstable. The first population is in rapid exchange with monomers and held together by electrostatic interactions; the second is kinetically more stable, dominates at later times, and is probably off-pathway to fibril formation. These more stable oligomers may contribute to other oligomer induced effects in the cellular environment, for example, by overloading protein quality control systems. We also show that the shortest growing filaments remain suspended in aqueous buffer and thus comprise a third, smaller population of transient oligomers with cross-β structure. Overall our data show that a diverse population of oligomers of different structures and half-lives are formed during the aggregation reaction with the great majority of oligomers formed not going on to form fibrils.
中文翻译:

Tau重复区聚集期间的低聚物多样性。
由于淀粉样蛋白聚集体与许多神经退行性疾病有关,因此蛋白质聚集的分子机制具有重要的基础和临床意义。这样的蛋白质聚集体包括宏观上不溶的原纤维以及小的可溶的寡聚物质。这些物种的时间依赖性分辨率是对蛋白质聚集进行详细定量了解的前提。由于缺乏检测和表征瞬时和异源蛋白寡聚体的方法,这仍然具有挑战性。在这里,我们已经使用单分子荧光技术结合机理建模来研究肝素诱导的tau重复区域的聚集,该区域形成了阿尔茨海默氏病中神经原纤维缠结的核心区域。我们区分了具有不同稳定性的寡聚体的几个亚群,并在聚集反应中根据温度和浓度的变化跟踪它们的演变。利用化学动力学技术揭示了两个最大的族群在结构上与原纤维不同,并且在动力学和热力学上都是不稳定的。第一族与单体快速交换并通过静电相互作用保持在一起。第二个在动力学上更稳定,在以后占主导地位,并且可能偏离了原纤维的形成。这些更稳定的寡聚物可能会在细胞环境中,例如通过使蛋白质质量控制系统超负荷,导致其他寡聚物诱导的效应。我们还表明,生长最短的细丝保持悬浮在水性缓冲液中,因此包含具有交叉β结构的第三种较小的瞬时低聚物群体。总的来说,我们的数据表明,在聚集反应期间形成了具有不同结构和半衰期的多种低聚物,其中形成的大多数低聚物不会继续形成原纤维。
更新日期:2018-06-28
中文翻译:

Tau重复区聚集期间的低聚物多样性。
由于淀粉样蛋白聚集体与许多神经退行性疾病有关,因此蛋白质聚集的分子机制具有重要的基础和临床意义。这样的蛋白质聚集体包括宏观上不溶的原纤维以及小的可溶的寡聚物质。这些物种的时间依赖性分辨率是对蛋白质聚集进行详细定量了解的前提。由于缺乏检测和表征瞬时和异源蛋白寡聚体的方法,这仍然具有挑战性。在这里,我们已经使用单分子荧光技术结合机理建模来研究肝素诱导的tau重复区域的聚集,该区域形成了阿尔茨海默氏病中神经原纤维缠结的核心区域。我们区分了具有不同稳定性的寡聚体的几个亚群,并在聚集反应中根据温度和浓度的变化跟踪它们的演变。利用化学动力学技术揭示了两个最大的族群在结构上与原纤维不同,并且在动力学和热力学上都是不稳定的。第一族与单体快速交换并通过静电相互作用保持在一起。第二个在动力学上更稳定,在以后占主导地位,并且可能偏离了原纤维的形成。这些更稳定的寡聚物可能会在细胞环境中,例如通过使蛋白质质量控制系统超负荷,导致其他寡聚物诱导的效应。我们还表明,生长最短的细丝保持悬浮在水性缓冲液中,因此包含具有交叉β结构的第三种较小的瞬时低聚物群体。总的来说,我们的数据表明,在聚集反应期间形成了具有不同结构和半衰期的多种低聚物,其中形成的大多数低聚物不会继续形成原纤维。



























 京公网安备 11010802027423号
京公网安备 11010802027423号