当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective total synthesis of pyrrolo-[2,1-c][1,4]-benzodiazepine monomers (S)-(−)-barmumycin and (S)-(+)-boseongazepine B†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1039/c8qo00446c Viraj A. Bhosale 1, 2, 3, 4 , Suresh B. Waghmode 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1039/c8qo00446c Viraj A. Bhosale 1, 2, 3, 4 , Suresh B. Waghmode 1, 2, 3, 4
Affiliation
|
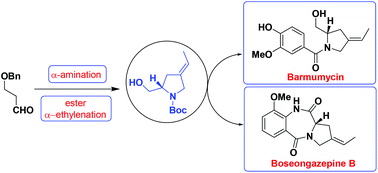
An efficient enantioselective total synthesis of pyrrolo-[2,1-c][1,4]benzodiazepine (PBD) monomers (S)-(−)-barmumycin and (S)-(+)-boseongazepine B and collective formal total syntheses of oxoprothracarcin, prothracarcin and (S)-(+)-boseongazepine C are described. The present approach is based on an efficient construction of an ethylidene substituted C-4 pyrrolidine core, that is the stereocontrolled introduction of a trisubstituted double bond through simple enolate α-alkylation of an ester, which also relies on a proline catalysed asymmetric α-amination followed by HWE olefination. The present synthetic route possesses superior stereocontrol over the C-4 ethylidene substituent as well as the C-(S) stereogenic center, which allows more functional variations on the five-membered prolinol core as compared to the existing PBD synthesis.
中文翻译:

对映体全合成吡咯并[[2,1- c ] [1,4]-苯并二氮杂monomer单体(S)-(-)-巴莫霉素和(S)-(+)-波塞加西平B †
吡咯并[[2,1- c ] [1,4]苯并二氮杂(PBD)单体(S)-(-)-巴莫霉素和(S)-(+)-boseongazepine B的有效对映选择性全合成和集体形式总合成记载了氧代原前列腺素,原前列腺素和(S)-(+)-波塞加西平C的化合物。本方法基于亚乙基取代的C-4吡咯烷核的有效构建,即通过酯的简单烯酸酯α-烷基化立体控制引入的三取代双键,这也依赖于脯氨酸催化的不对称α-胺化然后进行HWE烯烃化。本合成路线对C-4亚乙基取代基以及C-(S)立体生成中心,与现有的PBD合成方法相比,它在五元脯氨醇核心上具有更多功能上的变化。
更新日期:2018-06-29
中文翻译:

对映体全合成吡咯并[[2,1- c ] [1,4]-苯并二氮杂monomer单体(S)-(-)-巴莫霉素和(S)-(+)-波塞加西平B †
吡咯并[[2,1- c ] [1,4]苯并二氮杂(PBD)单体(S)-(-)-巴莫霉素和(S)-(+)-boseongazepine B的有效对映选择性全合成和集体形式总合成记载了氧代原前列腺素,原前列腺素和(S)-(+)-波塞加西平C的化合物。本方法基于亚乙基取代的C-4吡咯烷核的有效构建,即通过酯的简单烯酸酯α-烷基化立体控制引入的三取代双键,这也依赖于脯氨酸催化的不对称α-胺化然后进行HWE烯烃化。本合成路线对C-4亚乙基取代基以及C-(S)立体生成中心,与现有的PBD合成方法相比,它在五元脯氨醇核心上具有更多功能上的变化。










































 京公网安备 11010802027423号
京公网安备 11010802027423号