当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly efficient synthesis of C3-symmetric O-alkyl substituted triphenylenes and related Mannich derivatives†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1039/c8qo00414e Giacomo Berton 1, 2, 3, 4 , Giuseppe Borsato 1, 2, 3, 4 , Roberta Zangrando 3, 4, 5, 6 , Andrea Gambaro 2, 3, 4, 7, 8 , Fabrizio Fabris 1, 2, 3, 4 , Alessandro Scarso 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1039/c8qo00414e Giacomo Berton 1, 2, 3, 4 , Giuseppe Borsato 1, 2, 3, 4 , Roberta Zangrando 3, 4, 5, 6 , Andrea Gambaro 2, 3, 4, 7, 8 , Fabrizio Fabris 1, 2, 3, 4 , Alessandro Scarso 1, 2, 3, 4
Affiliation
|
C
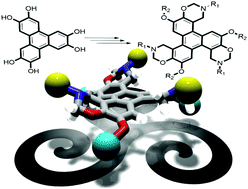
3-Symmetric tris-benzyl-O-substituted hexahydroxytriphenylene (HHTP) was prepared through selective ring opening with DIBAL-H in 48% yield (38% from HHTP in a two-step synthesis) avoiding the use of noxious, expensive and limited market availability reagents, with complete recovery of the undesired Cs co-product that is quantitatively recovered and converted back into HHTP. The C3-symmetric triphenylene product was further functionalized through substitution, deprotection and Mannich condensation reactions affording a series of C3-symmetric functionalized scaffolds in good yields for supramolecular applications.
中文翻译:

高效合成C 3对称的O-烷基取代的三亚苯基和相关的曼尼希衍生物†
通过用DIBAL-H选择性开环制备C 3-对称的三-苄基-O-取代的六羟基联苯(HHTP),产率为48%(两步合成中HHTP为38%),避免了使用有毒,昂贵且受限制的物质市场上可买到的试剂,可以完全回收不需要的C s副产物,并定量回收并转化回HHTP。通过取代,脱保护和曼尼希缩合反应将C 3对称的三亚苯基产物进一步官能化,得到一系列C 3对称的功能化支架,产量高,适用于超分子应用。
更新日期:2018-06-28
中文翻译:

高效合成C 3对称的O-烷基取代的三亚苯基和相关的曼尼希衍生物†
通过用DIBAL-H选择性开环制备C 3-对称的三-苄基-O-取代的六羟基联苯(HHTP),产率为48%(两步合成中HHTP为38%),避免了使用有毒,昂贵且受限制的物质市场上可买到的试剂,可以完全回收不需要的C s副产物,并定量回收并转化回HHTP。通过取代,脱保护和曼尼希缩合反应将C 3对称的三亚苯基产物进一步官能化,得到一系列C 3对称的功能化支架,产量高,适用于超分子应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号