当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
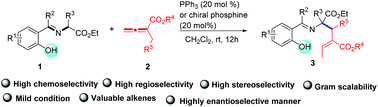
Hydroxy-assisted regio- and stereoselective synthesis of functionalized alkenes via phosphine-catalyzed β′-umpolung addition of o-hydroxy aromatic aldimines to allenoates†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1039/c8qo00460a Kunpeng Cheng 1, 2, 3, 4, 5 , Yishu Bao 1, 2, 3, 4, 5 , Jiahua Wang 1, 2, 3, 4, 5 , Zonghao Dai 1, 2, 3, 4, 5 , Jinrong Lu 1, 2, 3, 4, 5 , Qingfa Zhou 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1039/c8qo00460a Kunpeng Cheng 1, 2, 3, 4, 5 , Yishu Bao 1, 2, 3, 4, 5 , Jiahua Wang 1, 2, 3, 4, 5 , Zonghao Dai 1, 2, 3, 4, 5 , Jinrong Lu 1, 2, 3, 4, 5 , Qingfa Zhou 1, 2, 3, 4, 5
Affiliation
|
An unprecedented β′-umpolung addition of o-hydroxy aromatic aldimines with allenoates has been developed under the catalysis of phosphine. The reaction provides an efficient approach to the synthesis of functionalized alkenes in moderate to good yields with high stereoselectivities under mild conditions. The asymmetric version of this β′-umpolung addition has also been studied using chiral phosphines.
中文翻译:

羟基辅助官能化的烯烃的区域选择性和立体选择性合成经由膦催化的β'-极性转换加入ö羟基芳族醛亚胺到联烯酸酯†
在膦的催化作用下,已开发出空前的β-聚戊二烯酸与脲基甲酸酯的邻羟基芳族醛亚胺加成物。该反应为在温和条件下以中等至良好的产率,高的立体选择性合成官能化的烯烃提供了一种有效的方法。也已使用手性膦研究了这种β'-蛋白环加成物的不对称形式。
更新日期:2018-06-28
中文翻译:

羟基辅助官能化的烯烃的区域选择性和立体选择性合成经由膦催化的β'-极性转换加入ö羟基芳族醛亚胺到联烯酸酯†
在膦的催化作用下,已开发出空前的β-聚戊二烯酸与脲基甲酸酯的邻羟基芳族醛亚胺加成物。该反应为在温和条件下以中等至良好的产率,高的立体选择性合成官能化的烯烃提供了一种有效的方法。也已使用手性膦研究了这种β'-蛋白环加成物的不对称形式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号