当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deactivation of bimetallic nickel–copper alloy catalysts in thermocatalytic decomposition of methane†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1039/c8cy00339d Yi Shen 1, 2, 3, 4 , Moyan Ge 1, 2, 3, 4 , Aik Chong Lua 5, 6, 7
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1039/c8cy00339d Yi Shen 1, 2, 3, 4 , Moyan Ge 1, 2, 3, 4 , Aik Chong Lua 5, 6, 7
Affiliation
|
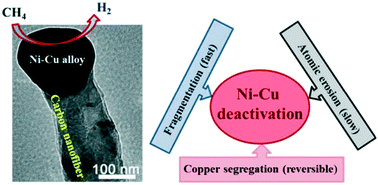
Bimetallic alloys are extensively used as catalysts because of their superior activity relative to the single constituent. Unfortunately, bimetallic catalysts suffer from complex deactivation. It is fundamentally important, but always highly challenging, to clearly elucidate the deactivation mechanism because of its complexity. Herein, the authors have endeavored to present a detailed study on the deactivation of Ni–Cu alloy catalysts supported on carbon nanotubes (Ni–Cu/CNT) for the thermocatalytic decomposition of methane. The activity of the catalysts was examined under two temperature-programmed modes, namely, a constant temperature mode and a cyclic heating–cooling temperature mode. The methane conversion, carbon deposition rate and carbon yield of the catalyst were recorded as a function of time. The structures of the resulting filamentous carbons were characterized by transmission electron microscopy, Raman spectroscopy and N2 adsorption–desorption isotherm analysis. The effects of the reaction temperature and copper content on the methane conversion, lifespan and carbon yield of the catalyst were investigated. The structural properties of the resulting carbons were correlated with the deactivation mechanism. It was found that the deactivation of the Ni–Cu/CNT catalysts is rather complex, which involves three deactivation mechanisms – atomic erosion, fragmentation and copper segregation. Under the constant temperature mode, the deactivation of the Ni–Cu/CNT catalysts is mainly attributed to the fragmentation of Ni–Cu nanoparticles at temperatures over 700 °C while atomic erosion plays a dominant role in the catalyst deactivation when the working temperature is less than 700 °C. Under the cyclic heating–cooling temperature mode, the activity of the deactivated Ni–Cu/CNT catalysts can be partially restored due to the re-establishment of the alloy surface composition after cooling to room temperature. Meanwhile, fragmentation of the Ni–Cu alloy also takes place under the cyclic heating–cooling temperature mode, which is responsible for the activity decay at each cycle and eventual catalyst deactivation.
中文翻译:

甲烷热催化分解中双金属镍-铜合金催化剂的失活†
双金属合金由于其相对于单一组分的优越活性而被广泛用作催化剂。不幸的是,双金属催化剂遭受复杂的失活。由于其复杂性,清楚地阐明停用机制从根本上很重要,但始终具有很高的挑战性。在本文中,作者努力进行了详细研究,研究了碳纳米管(Ni-Cu / CNT)上负载的Ni-Cu合金催化剂对甲烷的热催化分解的失活作用。在两种程序升温模式下检查了催化剂的活性,即恒温模式和循环加热-冷却温度模式。记录催化剂的甲烷转化率,碳沉积速率和碳产率与时间的关系。2个吸附-解吸等温线分析。研究了反应温度和铜含量对催化剂甲烷转化率,寿命和碳收率的影响。所得碳的结构性质与失活机理相关。研究发现,Ni-Cu / CNT催化剂的失活非常复杂,涉及三种失活机理:原子腐蚀,碎裂和铜偏析。在恒温模式下,Ni-Cu / CNT催化剂的失活主要归因于超过700°C的温度下Ni-Cu纳米颗粒的破碎,而当工作温度较低时,原子侵蚀在催化剂的失活中起主要作用高于700°C。在循环加热-冷却温度模式下,冷却至室温后,由于合金表面成分的重新建立,失活的Ni-Cu / CNT催化剂的活性可以部分恢复。同时,在循环加热-冷却温度模式下,Ni-Cu合金也发生碎裂,这导致每个循环的活性下降并最终使催化剂失活。
更新日期:2018-06-29
中文翻译:

甲烷热催化分解中双金属镍-铜合金催化剂的失活†
双金属合金由于其相对于单一组分的优越活性而被广泛用作催化剂。不幸的是,双金属催化剂遭受复杂的失活。由于其复杂性,清楚地阐明停用机制从根本上很重要,但始终具有很高的挑战性。在本文中,作者努力进行了详细研究,研究了碳纳米管(Ni-Cu / CNT)上负载的Ni-Cu合金催化剂对甲烷的热催化分解的失活作用。在两种程序升温模式下检查了催化剂的活性,即恒温模式和循环加热-冷却温度模式。记录催化剂的甲烷转化率,碳沉积速率和碳产率与时间的关系。2个吸附-解吸等温线分析。研究了反应温度和铜含量对催化剂甲烷转化率,寿命和碳收率的影响。所得碳的结构性质与失活机理相关。研究发现,Ni-Cu / CNT催化剂的失活非常复杂,涉及三种失活机理:原子腐蚀,碎裂和铜偏析。在恒温模式下,Ni-Cu / CNT催化剂的失活主要归因于超过700°C的温度下Ni-Cu纳米颗粒的破碎,而当工作温度较低时,原子侵蚀在催化剂的失活中起主要作用高于700°C。在循环加热-冷却温度模式下,冷却至室温后,由于合金表面成分的重新建立,失活的Ni-Cu / CNT催化剂的活性可以部分恢复。同时,在循环加热-冷却温度模式下,Ni-Cu合金也发生碎裂,这导致每个循环的活性下降并最终使催化剂失活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号