当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantifying vapor transfer into evaporating ethanol drops in a humid atmosphere
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1039/c8cp02521e Yutaku Kita 1, 2, 3, 4, 5 , Yuya Okauchi 2, 3, 4, 5, 6 , Yuki Fukatani 4, 7 , Daniel Orejon 1, 2, 3, 4, 5 , Masamichi Kohno 2, 3, 4, 5, 6 , Yasuyuki Takata 1, 2, 3, 4, 5 , Khellil Sefiane 8, 9, 10, 11, 12
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-06-28 00:00:00 , DOI: 10.1039/c8cp02521e Yutaku Kita 1, 2, 3, 4, 5 , Yuya Okauchi 2, 3, 4, 5, 6 , Yuki Fukatani 4, 7 , Daniel Orejon 1, 2, 3, 4, 5 , Masamichi Kohno 2, 3, 4, 5, 6 , Yasuyuki Takata 1, 2, 3, 4, 5 , Khellil Sefiane 8, 9, 10, 11, 12
Affiliation
|
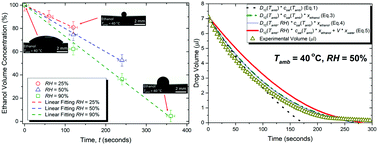
The effect of ambient temperature and relative humidity on the dynamics of ethanol drop evaporation is investigated. Although drop evaporation of mixtures and pure fluids has been extensively studied, very little is known about the transition from a pure fluid to a binary mixture following transfer of a second component present in the atmosphere. This is of importance for industrial, biological and medical applications where the purity of the solvent is paramount. Adsorption–absorption and/or condensation of water into ethanol drops during evaporation is presented through direct quantification of the drop composition in time. In particular, we combine drop profile measurements with Gas Injection Chromatography (GIC) to directly quantify the amount of ethanol evaporated and that of water intake over time. As expected, drops evaporate faster at higher temperatures since both the ethanol saturation concentration and the vapor diffusion coefficient are directly proportional to temperature. On the other hand, increases in the ethanol evaporation rate and in the water intake are observed at higher relative humidity. The increase in ethanol evaporation at higher relative humidity is interpreted by the greater diffusion coefficient of ethanol into humid air when compared to dry air. Moreover, as ethanol evaporates in a high humidity environment, the drop interfacial temperature falls below the dew point due to evaporative cooling and water condenses compared to lower humidity conditions. As a consequence of the heat released by adsorption–absorption and/or condensation, a greater temperature is reported at the liquid–vapor interface as confirmed by IR thermography, inducing a greater ethanol saturation concentration at the surface and hence a greater driving force for evaporation. By coupling the drop profile and the composition of ethanol and water within the drop, we propose a combined evaporation–adsorption/absorption and/or condensation empirical correlation. The proposed correlation accounts for: the decreases in ethanol concentration due to water adsorption–absorption and/or condensation, the diffusion coefficient dependence on relative humidity, and the amount of water intake during evaporation. The proposed empirical correlation agrees remarkably well with experimental observations.
中文翻译:

量化在潮湿气氛中转移到蒸发的乙醇滴中的蒸气
研究了环境温度和相对湿度对乙醇液滴蒸发动力学的影响。尽管已经对混合物和纯流体的液滴蒸发进行了广泛的研究,但对大气中存在的第二种组分转移后从纯流体到二元混合物的转变知之甚少。这对于溶剂纯度至关重要的工业,生物和医学应用非常重要。蒸发过程中水的吸附-吸收和/或凝结成乙醇滴是通过及时定量滴的成分来表示的。特别是,我们将液滴分布测量与气体注入色谱法(GIC)结合使用,可以直接量化随时间推移蒸发的乙醇量和取水量。不出所料 由于乙醇饱和浓度和蒸汽扩散系数均与温度成正比,因此在较高温度下,液滴的蒸发速度更快。另一方面,在较高的相对湿度下观察到乙醇蒸发速率和进水量的增加。与干燥空气相比,乙醇在较高的相对湿度下蒸发的增加是由乙醇向潮湿空气中的扩散系数更大所解释的。此外,随着乙醇在高湿度环境中蒸发,与较低湿度条件相比,由于蒸发冷却,界面温度下降到露点以下,并且水冷凝。由于吸附-吸收和/或冷凝过程中释放出的热量,据报道,红外-热成像技术证实了在液体-蒸汽界面的温度更高,在表面上引起更大的乙醇饱和浓度,并因此产生更大的蒸发驱动力。通过耦合液滴的分布以及液滴中乙醇和水的成分,我们提出了蒸发-吸附/吸收和/或冷凝的经验关系的组合。拟议的相关性解释如下:由于水的吸附-吸收和/或凝结导致乙醇浓度降低,扩散系数取决于相对湿度,以及蒸发过程中的进水量。所提出的经验相关性与实验观察结果非常吻合。我们提出了蒸发-吸附/吸收和/或冷凝的组合经验关系式。拟议的相关性解释如下:由于水的吸附-吸收和/或凝结导致乙醇浓度降低,扩散系数取决于相对湿度,以及蒸发过程中的进水量。所提出的经验相关性与实验观察结果非常吻合。我们提出了蒸发-吸附/吸收和/或冷凝的组合经验关系式。拟议的相关性解释如下:由于水的吸附-吸收和/或凝结导致乙醇浓度降低,扩散系数取决于相对湿度,以及蒸发过程中的进水量。所提出的经验相关性与实验观察结果非常吻合。
更新日期:2018-06-28
中文翻译:

量化在潮湿气氛中转移到蒸发的乙醇滴中的蒸气
研究了环境温度和相对湿度对乙醇液滴蒸发动力学的影响。尽管已经对混合物和纯流体的液滴蒸发进行了广泛的研究,但对大气中存在的第二种组分转移后从纯流体到二元混合物的转变知之甚少。这对于溶剂纯度至关重要的工业,生物和医学应用非常重要。蒸发过程中水的吸附-吸收和/或凝结成乙醇滴是通过及时定量滴的成分来表示的。特别是,我们将液滴分布测量与气体注入色谱法(GIC)结合使用,可以直接量化随时间推移蒸发的乙醇量和取水量。不出所料 由于乙醇饱和浓度和蒸汽扩散系数均与温度成正比,因此在较高温度下,液滴的蒸发速度更快。另一方面,在较高的相对湿度下观察到乙醇蒸发速率和进水量的增加。与干燥空气相比,乙醇在较高的相对湿度下蒸发的增加是由乙醇向潮湿空气中的扩散系数更大所解释的。此外,随着乙醇在高湿度环境中蒸发,与较低湿度条件相比,由于蒸发冷却,界面温度下降到露点以下,并且水冷凝。由于吸附-吸收和/或冷凝过程中释放出的热量,据报道,红外-热成像技术证实了在液体-蒸汽界面的温度更高,在表面上引起更大的乙醇饱和浓度,并因此产生更大的蒸发驱动力。通过耦合液滴的分布以及液滴中乙醇和水的成分,我们提出了蒸发-吸附/吸收和/或冷凝的经验关系的组合。拟议的相关性解释如下:由于水的吸附-吸收和/或凝结导致乙醇浓度降低,扩散系数取决于相对湿度,以及蒸发过程中的进水量。所提出的经验相关性与实验观察结果非常吻合。我们提出了蒸发-吸附/吸收和/或冷凝的组合经验关系式。拟议的相关性解释如下:由于水的吸附-吸收和/或凝结导致乙醇浓度降低,扩散系数取决于相对湿度,以及蒸发过程中的进水量。所提出的经验相关性与实验观察结果非常吻合。我们提出了蒸发-吸附/吸收和/或冷凝的组合经验关系式。拟议的相关性解释如下:由于水的吸附-吸收和/或凝结导致乙醇浓度降低,扩散系数取决于相对湿度,以及蒸发过程中的进水量。所提出的经验相关性与实验观察结果非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号