当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Size-dependent rate acceleration in the silylation of secondary alcohols: the bigger the faster†
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1039/c8sc01889h Marta Marin-Luna 1 , Benjamin Pölloth 1 , Fabian Zott 1 , Hendrik Zipse 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1039/c8sc01889h Marta Marin-Luna 1 , Benjamin Pölloth 1 , Fabian Zott 1 , Hendrik Zipse 1
Affiliation
|
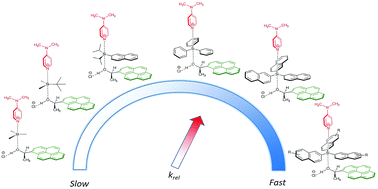
Relative rates for the reaction of secondary alcohols carrying large aromatic moieties with silyl chlorides carrying equally large substituents have been determined in organic solvents. Introducing thoroughly matching pairs of big dispersion energy donor (DED) groups enhanced rate constants up to four times, notably depending on the hydrogen bond donor ability of the solvent. A linear correlation between computed dispersion energy contributions to the stability of the silyl ether products and experimental relative rate constants was found. These results indicate a cooperation between solvophobic effects and DED-groups in the kinetic control of silylation reactions.
中文翻译:

仲醇硅烷化中尺寸依赖性的速率加速:越大越快†
带有大芳族部分的仲醇与带有同样大取代基的氯硅烷在有机溶剂中的反应相对速率已被确定。引入完全匹配的大色散能量供体 (DED) 基团对可将速率常数提高四倍,这主要取决于溶剂的氢键供体能力。计算的分散能对甲硅烷基醚产品稳定性的贡献与实验相对速率常数之间存在线性相关性。这些结果表明疏溶剂效应和 DED 基团在硅烷化反应的动力学控制中的协同作用。
更新日期:2018-06-29
中文翻译:

仲醇硅烷化中尺寸依赖性的速率加速:越大越快†
带有大芳族部分的仲醇与带有同样大取代基的氯硅烷在有机溶剂中的反应相对速率已被确定。引入完全匹配的大色散能量供体 (DED) 基团对可将速率常数提高四倍,这主要取决于溶剂的氢键供体能力。计算的分散能对甲硅烷基醚产品稳定性的贡献与实验相对速率常数之间存在线性相关性。这些结果表明疏溶剂效应和 DED 基团在硅烷化反应的动力学控制中的协同作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号