Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IL-23 secreted by myeloid cells drives castration-resistant prostate cancer
Nature ( IF 50.5 ) Pub Date : 2018-06-27 , DOI: 10.1038/s41586-018-0266-0 Arianna Calcinotto , Clarissa Spataro , Elena Zagato , Diletta Di Mitri , Veronica Gil , Mateus Crespo , Gaston De Bernardis , Marco Losa , Michela Mirenda , Emiliano Pasquini , Andrea Rinaldi , Semini Sumanasuriya , Maryou B. Lambros , Antje Neeb , Roberta Lucianò , Carlo A. Bravi , Daniel Nava-Rodrigues , David Dolling , Tommaso Prayer-Galetti , Ana Ferreira , Alberto Briganti , Antonio Esposito , Simon Barry , Wei Yuan , Adam Sharp , Johann de Bono , Andrea Alimonti
Nature ( IF 50.5 ) Pub Date : 2018-06-27 , DOI: 10.1038/s41586-018-0266-0 Arianna Calcinotto , Clarissa Spataro , Elena Zagato , Diletta Di Mitri , Veronica Gil , Mateus Crespo , Gaston De Bernardis , Marco Losa , Michela Mirenda , Emiliano Pasquini , Andrea Rinaldi , Semini Sumanasuriya , Maryou B. Lambros , Antje Neeb , Roberta Lucianò , Carlo A. Bravi , Daniel Nava-Rodrigues , David Dolling , Tommaso Prayer-Galetti , Ana Ferreira , Alberto Briganti , Antonio Esposito , Simon Barry , Wei Yuan , Adam Sharp , Johann de Bono , Andrea Alimonti
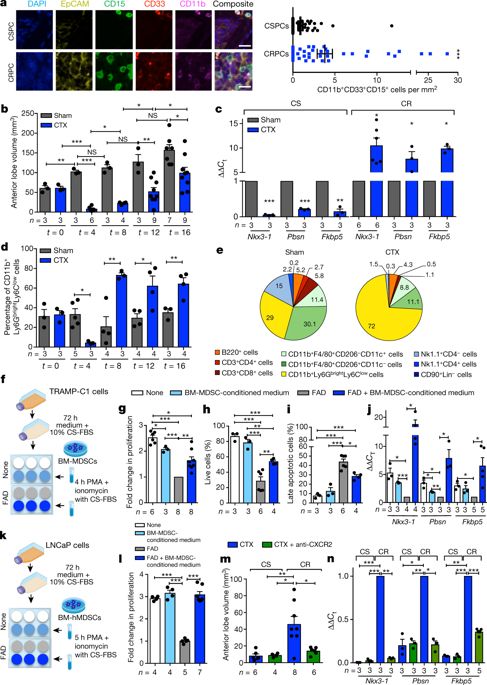
|
Patients with prostate cancer frequently show resistance to androgen-deprivation therapy, a condition known as castration-resistant prostate cancer (CRPC). Acquiring a better understanding of the mechanisms that control the development of CRPC remains an unmet clinical need. The well-established dependency of cancer cells on the tumour microenvironment indicates that the microenvironment might control the emergence of CRPC. Here we identify IL-23 produced by myeloid-derived suppressor cells (MDSCs) as a driver of CRPC in mice and patients with CRPC. Mechanistically, IL-23 secreted by MDSCs can activate the androgen receptor pathway in prostate tumour cells, promoting cell survival and proliferation in androgen-deprived conditions. Intra-tumour MDSC infiltration and IL-23 concentration are increased in blood and tumour samples from patients with CRPC. Antibody-mediated inactivation of IL-23 restored sensitivity to androgen-deprivation therapy in mice. Taken together, these results reveal that MDSCs promote CRPC by acting in a non-cell autonomous manner. Treatments that block IL-23 can oppose MDSC-mediated resistance to castration in prostate cancer and synergize with standard therapies.IL-23 produced by myeloid-derived suppressor cells regulates castration resistance in prostate cancer by sustaining androgen receptor signalling.
中文翻译:

骨髓细胞分泌的 IL-23 驱动去势抵抗性前列腺癌
前列腺癌患者经常表现出对雄激素剥夺疗法的抵抗力,这种情况称为去势抵抗性前列腺癌 (CRPC)。更好地了解控制 CRPC 发展的机制仍然是一个未满足的临床需求。癌细胞对肿瘤微环境的公认依赖性表明微环境可能控制 CRPC 的出现。在这里,我们将髓源性抑制细胞 (MDSC) 产生的 IL-23 鉴定为小鼠和 CRPC 患者中 CRPC 的驱动因素。从机制上讲,MDSCs 分泌的 IL-23 可以激活前列腺肿瘤细胞中的雄激素受体通路,促进细胞在雄激素缺乏条件下的存活和增殖。在 CRPC 患者的血液和肿瘤样本中,肿瘤内 MDSC 浸润和 IL-23 浓度增加。抗体介导的 IL-23 失活恢复了小鼠对雄激素剥夺疗法的敏感性。总之,这些结果表明 MDSCs 通过以非细胞自主方式起作用来促进 CRPC。阻断 IL-23 的治疗可以对抗 MDSC 介导的前列腺癌去势抵抗,并与标准疗法协同作用。 髓源性抑制细胞产生的 IL-23 通过维持雄激素受体信号传导来调节前列腺癌的去势抵抗。
更新日期:2018-06-27
中文翻译:

骨髓细胞分泌的 IL-23 驱动去势抵抗性前列腺癌
前列腺癌患者经常表现出对雄激素剥夺疗法的抵抗力,这种情况称为去势抵抗性前列腺癌 (CRPC)。更好地了解控制 CRPC 发展的机制仍然是一个未满足的临床需求。癌细胞对肿瘤微环境的公认依赖性表明微环境可能控制 CRPC 的出现。在这里,我们将髓源性抑制细胞 (MDSC) 产生的 IL-23 鉴定为小鼠和 CRPC 患者中 CRPC 的驱动因素。从机制上讲,MDSCs 分泌的 IL-23 可以激活前列腺肿瘤细胞中的雄激素受体通路,促进细胞在雄激素缺乏条件下的存活和增殖。在 CRPC 患者的血液和肿瘤样本中,肿瘤内 MDSC 浸润和 IL-23 浓度增加。抗体介导的 IL-23 失活恢复了小鼠对雄激素剥夺疗法的敏感性。总之,这些结果表明 MDSCs 通过以非细胞自主方式起作用来促进 CRPC。阻断 IL-23 的治疗可以对抗 MDSC 介导的前列腺癌去势抵抗,并与标准疗法协同作用。 髓源性抑制细胞产生的 IL-23 通过维持雄激素受体信号传导来调节前列腺癌的去势抵抗。










































 京公网安备 11010802027423号
京公网安备 11010802027423号