Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acquired resistance to IDH inhibition through trans or cis dimer-interface mutations
Nature ( IF 50.5 ) Pub Date : 2018-06-27 , DOI: 10.1038/s41586-018-0251-7 Andrew M Intlekofer 1, 2, 3, 4 , Alan H Shih 1, 2, 4, 5 , Bo Wang 1, 2, 6 , Abbas Nazir 1, 2 , Ariën S Rustenburg 7 , Steven K Albanese 7, 8 , Minal Patel 2 , Christopher Famulare 2 , Fabian M Correa 1, 2 , Naofumi Takemoto 1, 2 , Vidushi Durani 1, 2 , Hui Liu 9 , Justin Taylor 1, 2, 4, 5 , Noushin Farnoud 2, 10, 11 , Elli Papaemmanuil 2, 10, 11 , Justin R Cross 9 , Martin S Tallman 4, 5 , Maria E Arcila 12 , Mikhail Roshal 12 , Gregory A Petsko 13 , Bin Wu 14 , Sung Choe 14 , Zenon D Konteatis 14 , Scott A Biller 14 , John D Chodera 7 , Craig B Thompson 6 , Ross L Levine 1, 2, 4, 5 , Eytan M Stein 4, 5
Nature ( IF 50.5 ) Pub Date : 2018-06-27 , DOI: 10.1038/s41586-018-0251-7 Andrew M Intlekofer 1, 2, 3, 4 , Alan H Shih 1, 2, 4, 5 , Bo Wang 1, 2, 6 , Abbas Nazir 1, 2 , Ariën S Rustenburg 7 , Steven K Albanese 7, 8 , Minal Patel 2 , Christopher Famulare 2 , Fabian M Correa 1, 2 , Naofumi Takemoto 1, 2 , Vidushi Durani 1, 2 , Hui Liu 9 , Justin Taylor 1, 2, 4, 5 , Noushin Farnoud 2, 10, 11 , Elli Papaemmanuil 2, 10, 11 , Justin R Cross 9 , Martin S Tallman 4, 5 , Maria E Arcila 12 , Mikhail Roshal 12 , Gregory A Petsko 13 , Bin Wu 14 , Sung Choe 14 , Zenon D Konteatis 14 , Scott A Biller 14 , John D Chodera 7 , Craig B Thompson 6 , Ross L Levine 1, 2, 4, 5 , Eytan M Stein 4, 5
Affiliation
|
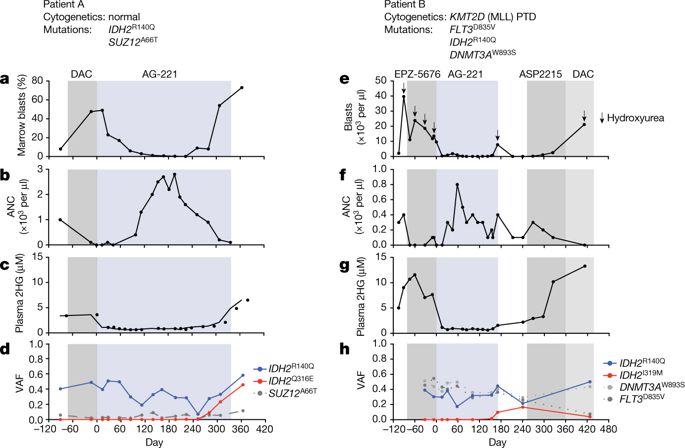
Somatic mutations in the isocitrate dehydrogenase 2 gene (IDH2) contribute to the pathogenesis of acute myeloid leukaemia (AML) through the production of the oncometabolite 2-hydroxyglutarate (2HG)1–8. Enasidenib (AG-221) is an allosteric inhibitor that binds to the IDH2 dimer interface and blocks the production of 2HG by IDH2 mutants9,10. In a phase I/II clinical trial, enasidenib inhibited the production of 2HG and induced clinical responses in relapsed or refractory IDH2-mutant AML11. Here we describe two patients with IDH2-mutant AML who had a clinical response to enasidenib followed by clinical resistance, disease progression, and a recurrent increase in circulating levels of 2HG. We show that therapeutic resistance is associated with the emergence of second-site IDH2 mutations in trans, such that the resistance mutations occurred in the IDH2 allele without the neomorphic R140Q mutation. The in trans mutations occurred at glutamine 316 (Q316E) and isoleucine 319 (I319M), which are at the interface where enasidenib binds to the IDH2 dimer. The expression of either of these mutant disease alleles alone did not induce the production of 2HG; however, the expression of the Q316E or I319M mutation together with the R140Q mutation in trans allowed 2HG production that was resistant to inhibition by enasidenib. Biochemical studies predicted that resistance to allosteric IDH inhibitors could also occur via IDH dimer-interface mutations in cis, which was confirmed in a patient with acquired resistance to the IDH1 inhibitor ivosidenib (AG-120). Our observations uncover a mechanism of acquired resistance to a targeted therapy and underscore the importance of 2HG production in the pathogenesis of IDH-mutant malignancies.A new mechanism of acquired clinical resistance in two patients with acute myeloid leukaemia driven by mutant IDH2 is described, in which a second-site mutation on the wild-type allele induces therapeutic resistance to IDH2 inhibitors.
中文翻译:

通过反式或顺式二聚体界面突变获得对 IDH 抑制的抗性
异柠檬酸脱氢酶 2 基因 (IDH2) 的体细胞突变通过产生致癌代谢物 2-羟基戊二酸 (2HG)1-8 促进急性髓细胞白血病 (AML) 的发病机制。Enasidenib (AG-221) 是一种变构抑制剂,可与 IDH2 二聚体界面结合并阻断 IDH2 突变体 9,10 产生 2HG。在 I/II 期临床试验中,enasidenib 抑制 2HG 的产生并诱导复发或难治性 IDH2 突变 AML11 的临床反应。在这里,我们描述了两名 IDH2 突变 AML 患者,他们对 enasidenib 有临床反应,随后出现临床耐药、疾病进展和循环 2HG 水平的反复增加。我们表明,治疗耐药与反式第二位点 IDH2 突变的出现有关,因此,耐药突变发生在 IDH2 等位基因中,而没有新的 R140Q 突变。反式突变发生在谷氨酰胺 316 (Q316E) 和异亮氨酸 319 (I319M),它们位于 enasidenib 与 IDH2 二聚体结合的界面处。这些突变疾病等位基因中的任何一个单独的表达都不会诱导2HG的产生。然而,Q316E 或 I319M 突变与反式 R140Q 突变的表达允许产生对 enasidenib 抑制具有抗性的 2HG。生化研究预测,对变构 IDH 抑制剂的耐药性也可能通过顺式中的 IDH 二聚体界面突变发生,这在对 IDH1 抑制剂 ivosidenib (AG-120) 获得性耐药的患者中得到证实。
更新日期:2018-06-27
中文翻译:

通过反式或顺式二聚体界面突变获得对 IDH 抑制的抗性
异柠檬酸脱氢酶 2 基因 (IDH2) 的体细胞突变通过产生致癌代谢物 2-羟基戊二酸 (2HG)1-8 促进急性髓细胞白血病 (AML) 的发病机制。Enasidenib (AG-221) 是一种变构抑制剂,可与 IDH2 二聚体界面结合并阻断 IDH2 突变体 9,10 产生 2HG。在 I/II 期临床试验中,enasidenib 抑制 2HG 的产生并诱导复发或难治性 IDH2 突变 AML11 的临床反应。在这里,我们描述了两名 IDH2 突变 AML 患者,他们对 enasidenib 有临床反应,随后出现临床耐药、疾病进展和循环 2HG 水平的反复增加。我们表明,治疗耐药与反式第二位点 IDH2 突变的出现有关,因此,耐药突变发生在 IDH2 等位基因中,而没有新的 R140Q 突变。反式突变发生在谷氨酰胺 316 (Q316E) 和异亮氨酸 319 (I319M),它们位于 enasidenib 与 IDH2 二聚体结合的界面处。这些突变疾病等位基因中的任何一个单独的表达都不会诱导2HG的产生。然而,Q316E 或 I319M 突变与反式 R140Q 突变的表达允许产生对 enasidenib 抑制具有抗性的 2HG。生化研究预测,对变构 IDH 抑制剂的耐药性也可能通过顺式中的 IDH 二聚体界面突变发生,这在对 IDH1 抑制剂 ivosidenib (AG-120) 获得性耐药的患者中得到证实。











































 京公网安备 11010802027423号
京公网安备 11010802027423号