当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Au(iii) and Pt(ii) complexes with Na/K-ATPase: experimental and theoretical study of reaction stoichiometry and binding sites†
Metallomics ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1039/c8mt00111a Ana V. Vujačić Nikezić 1, 2, 3, 4 , Goran V. Janjić 2, 3, 4, 5 , Aleksandra M. Bondžić 1, 2, 3, 4 , Božidarka L. Zarić 1, 2, 3, 4 , Dragana D. Vasić-Anićijević 1, 2, 3, 4 , Tatjana G. Momić 1, 2, 3, 4 , Vesna M. Vasić 1, 2, 3, 4
Metallomics ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1039/c8mt00111a Ana V. Vujačić Nikezić 1, 2, 3, 4 , Goran V. Janjić 2, 3, 4, 5 , Aleksandra M. Bondžić 1, 2, 3, 4 , Božidarka L. Zarić 1, 2, 3, 4 , Dragana D. Vasić-Anićijević 1, 2, 3, 4 , Tatjana G. Momić 1, 2, 3, 4 , Vesna M. Vasić 1, 2, 3, 4
Affiliation
|
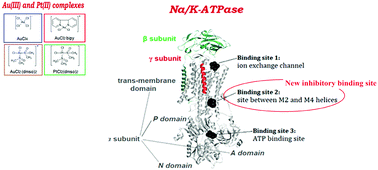
The present paper deals with investigation of the interaction between selected simple structure Au(III) ([AuCl4]−, [AuCl2(dmso)2]+, [AuCl2(bipy)]+) and Pt(II) ([PtCl2(dmso)2]) complexes with Na/K-ATPase as the target enzyme, using an experimental and theoretical approach. Reaction stoichiometries and binding constants for these enzyme/complex systems were determined, while kinetic measurements were used in order to reveal the type of inhibition. Based on the results obtained by quantum mechanical calculations (electrostatic surface potential (ESP), volume and surface of the complexes) the nature of the investigated complexes was characterized. By using the solvent accessible surface area (SASA) applied on specific inhibitory sites (ion channel and intracellular domains) the nature of these sites was described. Docking studies were used to determine the theoretical probability of the non-covalent metal binding site positions. Inhibition studies implied that all the investigated complexes decreased the activity of the enzyme while the kinetic analysis indicated an uncompetitive mode of inhibition for the selected complexes. Docking results suggested that the main inhibitory site of all these complexes is located in the ion translocation pathway on the extracellular side in the E2P enzyme conformation, similar to the case of cardiac glycosides, specific Na/K-ATPase inhibitors. Also, based on our knowledge, the hydrolyzed forms of [AuCl4]− and [PtCl2(dmso)2] complexes were investigated for the first time by theoretical calculations in this paper. Thereby, a new inhibitory site situated between the M2 and M4 helices was revealed. Binding in this site induces conformational changes in the enzyme domains and perturbs the E1–E2P conformational equilibrium, causing enzyme inhibition.
中文翻译:

Au(iii)和Pt(ii)配合物与Na / K-ATPase的相互作用:反应化学计量和结合位点的实验和理论研究†
本文研究选定的简单结构Au(III)([AuCl 4 ] -,[AuCl 2(dmso)2 ] +,[AuCl 2(bipy)] +)和Pt(II)([ PtCl 2(dmso)2])使用实验和理论方法,将Na / K-ATPase作为目标酶复合物。确定了这些酶/复合系统的反应化学计量和结合常数,同时使用动力学测量来揭示抑制的类型。基于通过量子力学计算获得的结果(静电表面电势(ESP),配合物的体积和表面),对所研究配合物的性质进行了表征。通过使用应用于特定抑制位点(离子通道和细胞内结构域)的溶剂可及表面积(SASA),描述了这些位点的性质。对接研究用于确定非共价金属结合位点位置的理论概率。抑制研究表明,所有研究的复合物均降低了酶的活性,而动力学分析表明,对所选复合物的抑制方式是非竞争性的。对接结果表明,所有这些复合物的主要抑制位点都位于E2P酶构象的细胞外侧的离子易位途径中,类似于强心苷,即特定的Na / K-ATPase抑制剂。同样,基于我们的知识,[AuCl 特定的Na / K-ATPase抑制剂。同样,基于我们的知识,[AuCl 特定的Na / K-ATPase抑制剂。同样,基于我们的知识,[AuCl本文通过理论计算首次研究了4 ] -和[PtCl 2(dmso) 2 ]配合物。由此,揭示了位于M2和M4螺旋之间的新的抑制位点。在该位点的结合诱导了酶结构域的构象变化,并扰乱了E1-E2P构象平衡,从而导致了酶的抑制。
更新日期:2018-06-27
中文翻译:

Au(iii)和Pt(ii)配合物与Na / K-ATPase的相互作用:反应化学计量和结合位点的实验和理论研究†
本文研究选定的简单结构Au(III)([AuCl 4 ] -,[AuCl 2(dmso)2 ] +,[AuCl 2(bipy)] +)和Pt(II)([ PtCl 2(dmso)2])使用实验和理论方法,将Na / K-ATPase作为目标酶复合物。确定了这些酶/复合系统的反应化学计量和结合常数,同时使用动力学测量来揭示抑制的类型。基于通过量子力学计算获得的结果(静电表面电势(ESP),配合物的体积和表面),对所研究配合物的性质进行了表征。通过使用应用于特定抑制位点(离子通道和细胞内结构域)的溶剂可及表面积(SASA),描述了这些位点的性质。对接研究用于确定非共价金属结合位点位置的理论概率。抑制研究表明,所有研究的复合物均降低了酶的活性,而动力学分析表明,对所选复合物的抑制方式是非竞争性的。对接结果表明,所有这些复合物的主要抑制位点都位于E2P酶构象的细胞外侧的离子易位途径中,类似于强心苷,即特定的Na / K-ATPase抑制剂。同样,基于我们的知识,[AuCl 特定的Na / K-ATPase抑制剂。同样,基于我们的知识,[AuCl 特定的Na / K-ATPase抑制剂。同样,基于我们的知识,[AuCl本文通过理论计算首次研究了4 ] -和[PtCl 2(dmso) 2 ]配合物。由此,揭示了位于M2和M4螺旋之间的新的抑制位点。在该位点的结合诱导了酶结构域的构象变化,并扰乱了E1-E2P构象平衡,从而导致了酶的抑制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号