Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-06-27 , DOI: 10.1016/j.jcat.2018.06.005 Jing Li , Zhishan Su , Junming Wang , Changwei Hu
|
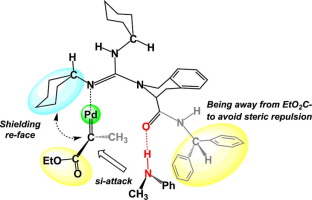
The mechanism and stereoselectivity of the asymmetric N-H insertion reactions between α-diazocarbonyl compounds and amines mediated by palladium-chiral guanidine complexes were investigated at the BP86-D3(BJ)/def2TZVP (SMD, CH2Cl2)//BP86-D3(BJ)/def2SVP (SMD, CH2Cl2) level at 303 K. The non-catalytic reaction occurred through a stepwise mechanism, with a high activation barrier of 56.4 kcal mol−1. Good linear correlations between the global nucleophilicity index (N) of amine, Hammett substituent constants (σP), and the activation energy barriers (ΔG≠) were found. The Pd(0)-guanidine-catalyzed reaction consisted of three continuous steps, including: (i) generation of Pd-carbene intermediate by dinitrogen loss from α-diazoesters substrate, (ii) formation of CN bond, and (iii) 1,2-H transfer by metal-associated ylide, accompanying with the regeneration of catalyst. A water molecule accelerated the final H-transfer by constructing hydrogen bonding network. The cyclohexyl group in ligand provided sufficient steric shielding around Pd-carbene intermediate from the re-face attack by amines. The combination of the hydrogen bonding orientation of amide moiety of guanidine ligand, as well as the steric repulsion between the ester group of α-diazoester substrate and bulky
CH(Ph)2 group in ligand played an important role in controlling the stereoselectivity, affording the predominant S-configuration product observed in experiment. Introducing one aromatic ring to chiral backbone of the guanidine ligand enhanced the enantiodifferentiation of products by increasing the difference of strain energy (ΔΔEstrain) of Pd-carbene moiety along two competing pathways. Different from Pd(0)-catalyst, the Pd(II)-chiral guanidine complex accelerated N-H insertion reaction via Lewis acid catalysis. In this process, the formation of free ylide in the reaction led to low ee. These results were in good agreement with experimental observations.
中文翻译:

钯-手性胍配合物催化胺不对称NH插入的机理研究
在BP86-D3(BJ)/ def2TZVP(SMD,CH 2 Cl 2)// BP86-D3( BJ)/ def2SVP(SMD,CH 2 Cl 2)的水平在303K。非催化反应通过逐步机制发生,具有56.4 kcal mol -1的高活化势垒。全局亲核性指数(之间具有良好的线性关系Ñ胺,哈米特取代基常数(σ)的P),且活化能障碍(Δ ģ ≠) 被发现。Pd(0)-胍催化的反应包括三个连续步骤,包括:(i)通过从二重氮基酯底物损失二氮来生成Pd-卡宾中间体,(ii)形成C N键,以及(iii)1通过金属缔合的叶立德转移2-2 - H,并伴随催化剂的再生。水分子通过构建氢键网络加快了最终的H转移。在配位体的环己基周围的Pd-卡宾从中间提供了足够的空间屏蔽再通过胺-面攻击。胍配体酰胺部分的氢键键合方向,以及α-重氮酯底物的酯基与大分子
CH(Ph)2之间的空间排斥力配体中的基团在控制立体选择性中起重要作用,提供了实验中观察到的主要S构型产物。引入一个芳香环的胍的手性配体骨架通过增加应变能量的差(ΔΔ提高了产品enantiodifferentiation ë应变沿着两个竞争性途径的Pd-碳烯部分的)。与Pd(0)催化剂不同,Pd(II)-手性胍络合物通过路易斯酸催化加速NH插入反应。在此过程中,反应中游离叶立德的形成导致ee低。这些结果与实验观察结果非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号