当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and reactivity of a nickel(ii) thioperoxide complex: demonstration of sulfide-mediated N2O reduction†
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1039/c8sc02536c Nathaniel J Hartmann 1 , Guang Wu 1 , Trevor W Hayton 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1039/c8sc02536c Nathaniel J Hartmann 1 , Guang Wu 1 , Trevor W Hayton 1
Affiliation
|
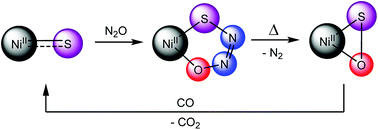
The thiohyponitrite ([SNNO]2−) complex, [K(18-crown-6)][LtBuNiII(κ2-SNNO)] (LtBu = {(2,6-iPr2C6H3)NC(tBu)}2CH), extrudes N2 under mild heating to yield [K(18-crown-6)][LtBuNiII(η2-SO)] (1), along with minor products [K(18-crown-6)][LtBuNiII(η2-OSSO)] (2) and [K(18-crown-6)][LtBuNiII(η2-S2)] (3). Subsequent reaction of 1 with carbon monoxide (CO) results in the formation of [K(18-crown-6)][LtBuNiII(η2-SCO)] (4), [K(18-crown-6)][LtBuNiII(S,O:κ2-SCO2)] (5), [K(18-crown-6)][LtBuNiII(κ2-CO3)] (6), carbonyl sulfide (COS) (7), and [K(18-crown-6)][LtBuNiII(S2CO)] (8). To rationalize the formation of these products we propose that 1 first reacts with CO to form [K(18-crown-6)][LtBuNiII(S)] (I) and CO2, via O-atom abstraction. Subsequently, complex I reacts with CO or CO2 to form 4 and 5, respectively. Similarly, the formation of complex 6 and COS can be rationalized by the reaction of 1 with CO2 to form a putative Ni(II) monothiopercarbonate, [K(18-crown-6)][LtBuNiII(κ2-SOCO2)] (11). The Ni(II) monothiopercarbonate subsequently transfers a S-atom to CO to form COS and [K(18-crown-6)][LtBuNiII(κ2-CO3)] (6). Finally, the formation of 8 can be rationalized by the reaction of COS with I. Critically, the observation of complexes 4 and 5 in the reaction mixture reveals the stepwise conversion of [K(18-crown-6)][LtBuNiII(κ2-SNNO)] to 1 and then I, which represents the formal reduction of N2O by CO.
中文翻译:

镍 (ii) 硫过氧化物络合物的合成和反应性:硫化物介导的 N2O 还原的示范†
硫代次亚硝酸盐 ([SNNO] 2- ) 络合物 [K(18-crown-6)][L t Bu Ni II (κ 2 -SNNO)] (L t Bu = {(2,6- i Pr 2 C 6 H 3 )NC( t Bu)} 2 CH),在温和加热下挤出 N 2得到 [K(18-crown-6)][L t Bu Ni II (η 2 -SO)] ( 1 ),以及次要产品 [K(18-crown-6)][L t Bu Ni II (η 2 -OSSO)] ( 2) 和 [K(18-crown-6)][L t Bu Ni II (η 2 -S 2 )] ( 3 )。1与一氧化碳 (CO) 的后续反应导致形成 [K(18-crown-6)][L t Bu Ni II (η 2 -SCO)] ( 4 ), [K(18-crown-6) )][L t Bu Ni II ( S , O :κ 2 -SCO 2 )] ( 5 ), [K(18-crown-6)][L t Bu Ni II (κ 2 -CO 3 )] (6 )、羰基硫(COS) ( 7 )和[K(18-crown-6)][L t Bu Ni II (S 2 CO)] ( 8 )。为了使这些产物的形成合理化,我们建议1首先通过O 原子抽象与 CO 反应形成 [K(18-crown-6)][L t Bu Ni II (S)] ( I ) 和 CO 2 。随后,配合物I与 CO 或 CO 2反应分别形成4和5。同样,形成络合物6和 COS 可以通过1与 CO 2反应形成推定的 Ni( II ) 单硫代过碳酸酯 [K(18-crown-6)][L t Bu Ni II (κ 2 -SOCO 2 )] ( 11 )来合理化. Ni( II ) 单硫代过碳酸酯随后将 S 原子转移到 CO 以形成 COS 和 [K(18-crown-6)][L t Bu Ni II (κ 2 -CO 3 )] ( 6 )。最后,8的形成可以通过 COS 与I的反应来合理化。至关重要的是,观察配合物反应混合物中的图4和5揭示了 [K(18-crown-6)][L t Bu Ni II (κ 2 -SNNO)] 逐步转化为1然后是I,这代表 N 2 O的正式还原由 CO.
更新日期:2018-06-27
中文翻译:

镍 (ii) 硫过氧化物络合物的合成和反应性:硫化物介导的 N2O 还原的示范†
硫代次亚硝酸盐 ([SNNO] 2- ) 络合物 [K(18-crown-6)][L t Bu Ni II (κ 2 -SNNO)] (L t Bu = {(2,6- i Pr 2 C 6 H 3 )NC( t Bu)} 2 CH),在温和加热下挤出 N 2得到 [K(18-crown-6)][L t Bu Ni II (η 2 -SO)] ( 1 ),以及次要产品 [K(18-crown-6)][L t Bu Ni II (η 2 -OSSO)] ( 2) 和 [K(18-crown-6)][L t Bu Ni II (η 2 -S 2 )] ( 3 )。1与一氧化碳 (CO) 的后续反应导致形成 [K(18-crown-6)][L t Bu Ni II (η 2 -SCO)] ( 4 ), [K(18-crown-6) )][L t Bu Ni II ( S , O :κ 2 -SCO 2 )] ( 5 ), [K(18-crown-6)][L t Bu Ni II (κ 2 -CO 3 )] (6 )、羰基硫(COS) ( 7 )和[K(18-crown-6)][L t Bu Ni II (S 2 CO)] ( 8 )。为了使这些产物的形成合理化,我们建议1首先通过O 原子抽象与 CO 反应形成 [K(18-crown-6)][L t Bu Ni II (S)] ( I ) 和 CO 2 。随后,配合物I与 CO 或 CO 2反应分别形成4和5。同样,形成络合物6和 COS 可以通过1与 CO 2反应形成推定的 Ni( II ) 单硫代过碳酸酯 [K(18-crown-6)][L t Bu Ni II (κ 2 -SOCO 2 )] ( 11 )来合理化. Ni( II ) 单硫代过碳酸酯随后将 S 原子转移到 CO 以形成 COS 和 [K(18-crown-6)][L t Bu Ni II (κ 2 -CO 3 )] ( 6 )。最后,8的形成可以通过 COS 与I的反应来合理化。至关重要的是,观察配合物反应混合物中的图4和5揭示了 [K(18-crown-6)][L t Bu Ni II (κ 2 -SNNO)] 逐步转化为1然后是I,这代表 N 2 O的正式还原由 CO.











































 京公网安备 11010802027423号
京公网安备 11010802027423号