当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Correlation for ε-2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane in Different Alkanes/Aromatic Hydrocarbon + Ethyl Acetate Binary Solvents at Temperatures of between 283.15 and 323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-27 , DOI: 10.1021/acs.jced.8b00353 Chao Cui 1 , Hui Ren 1 , Qingjie Jiao 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-27 , DOI: 10.1021/acs.jced.8b00353 Chao Cui 1 , Hui Ren 1 , Qingjie Jiao 1
Affiliation
|
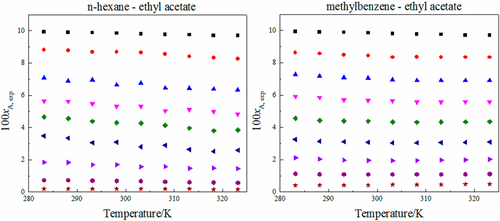
The solubility data of ε-2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane in six binary solvents (n-hexane, n-heptane, n-octane, isooctane, cyclohexane, and methylbenzene mixed with various mole fractions of ethyl acetate) was experimentally studied by the gravimetric method at temperatures ranging from 283.15 to 323.15 K under an atmosphere pressure of 0.1 MPa. The results of these measurements were correlated with the modified Apelblat equation, CNIBS/R-K equation, and Jouyban–Acree model. All three models provide a satisfactory correlation.
中文翻译:

ε-2,4,6,8,10,12-六硝基-2,4,6,8,10,12-六氮杂异纤锌矿型烷烃在温度为283.15之间的烷烃/芳烃+乙酸乙酯乙酸乙酯二元溶剂中的溶解度测量及相关性和323.15 K
ε-2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-和还原剂在六个二进制溶剂中的溶解度数据(Ñ己烷,Ñ -庚烷,Ñ辛烷,异辛烷,环己烷和甲基苯(与各种摩尔分数的乙酸乙酯混合)通过重量分析法在0.1 MPa的大气压下于283.15至323.15 K范围内进行了实验研究。这些测量结果与修改后的Apelblat方程,CNIBS / RK方程和Jouyban–Acree模型相关。所有这三个模型都提供了令人满意的相关性。
更新日期:2018-06-28
中文翻译:

ε-2,4,6,8,10,12-六硝基-2,4,6,8,10,12-六氮杂异纤锌矿型烷烃在温度为283.15之间的烷烃/芳烃+乙酸乙酯乙酸乙酯二元溶剂中的溶解度测量及相关性和323.15 K
ε-2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-和还原剂在六个二进制溶剂中的溶解度数据(Ñ己烷,Ñ -庚烷,Ñ辛烷,异辛烷,环己烷和甲基苯(与各种摩尔分数的乙酸乙酯混合)通过重量分析法在0.1 MPa的大气压下于283.15至323.15 K范围内进行了实验研究。这些测量结果与修改后的Apelblat方程,CNIBS / RK方程和Jouyban–Acree模型相关。所有这三个模型都提供了令人满意的相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号