当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generalized Born Based Continuous Constant pH Molecular Dynamics in Amber: Implementation, Benchmarking and Analysis
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.jcim.8b00227 Yandong Huang 1 , Robert C. Harris 1 , Jana Shen 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.jcim.8b00227 Yandong Huang 1 , Robert C. Harris 1 , Jana Shen 1
Affiliation
|
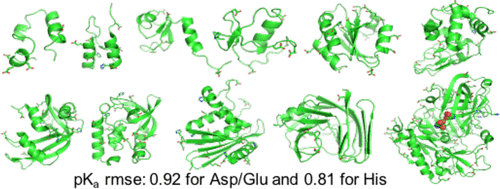
Solution pH plays an important role in structure and dynamics of biomolecular systems; however, pH effects cannot be accurately accounted for in conventional molecular dynamics simulations based on fixed protonation states. Continuous constant pH molecular dynamics (CpHMD) based on the λ-dynamics framework calculates protonation states on the fly during dynamical simulation at a specified pH condition. Here we report the CPU-based implementation of the CpHMD method based on the GBNeck2 generalized Born (GB) implicit-solvent model in the pmemd engine of the Amber molecular dynamics package. The performance of the method was tested using pH replica-exchange titration simulations of Asp, Glu and His side chains in 4 miniproteins and 7 enzymes with experimentally known pKa’s, some of which are significantly shifted from the model values. The added computational cost due to CpHMD titration ranges from 11 to 33% for the data set and scales roughly linearly as the ratio between the titrable sites and number of solute atoms. Comparison of the experimental and calculated pKa’s using 2 ns per replica sampling yielded a mean unsigned error of 0.70, a root-mean-squared error of 0.91, and a linear correlation coefficient of 0.79. Though this level of accuracy is similar to the GBSW-based CpHMD in CHARMM, in contrast to the latter, the current implementation was able to reproduce the experimental orders of the pKa’s of the coupled carboxylic dyads. We quantified the sampling errors, which revealed that prolonged simulation is needed to converge pKa’s of several titratable groups involved in salt-bridge-like interactions or deeply buried in the protein interior. Our benchmark data demonstrate that GBNeck2-CpHMD is an attractive tool for protein pKa predictions.
中文翻译:

琥珀色中基于广义Born的连续恒定pH分子动力学:实现,对标和分析
溶液的pH值在生物分子系统的结构和动力学中起着重要的作用。但是,在基于固定质子化状态的常规分子动力学模拟中,无法准确地解释pH值的影响。基于λ动力学框架的连续恒定pH分子动力学(CpHMD)可在指定pH条件下的动力学模拟过程中动态计算质子化状态。在这里,我们报告了琥珀色分子动力学软件包的pmemd引擎中基于GBNeck2广义Born(GB)隐式溶剂模型的CpHMD方法的基于CPU的实现。使用pH副本交换滴定模拟了实验已知的p K a的4种小蛋白和7种酶中的Asp,Glu和His侧链的方法,测试了方法的性能,其中一些与模型值大不相同。对于数据集,由于CpHMD滴定而导致的增加的计算成本在11%到33%的范围内,并且随着可滴定位点与溶质原子数之间的比率大致线性地缩放。将每个副本采样使用2 ns的实验值和计算出的p K a进行比较,得出的平均无符号误差为0.70,均方根误差为0.91,线性相关系数为0.79。尽管此精度水平与CHARMM中基于GBSW的CpHMD相似,但与后者相反,当前的实现方式能够重现p K a的实验顺序。是偶合的羧基二元化合物。我们对采样误差进行了量化,这表明需要长时间的模拟才能使参与盐桥样相互作用或深埋在蛋白质内部的几个可滴定基团的p K a收敛。我们的基准数据表明,GBNeck2-CpHMD是蛋白质p K a预测的有吸引力的工具。
更新日期:2018-06-27
中文翻译:

琥珀色中基于广义Born的连续恒定pH分子动力学:实现,对标和分析
溶液的pH值在生物分子系统的结构和动力学中起着重要的作用。但是,在基于固定质子化状态的常规分子动力学模拟中,无法准确地解释pH值的影响。基于λ动力学框架的连续恒定pH分子动力学(CpHMD)可在指定pH条件下的动力学模拟过程中动态计算质子化状态。在这里,我们报告了琥珀色分子动力学软件包的pmemd引擎中基于GBNeck2广义Born(GB)隐式溶剂模型的CpHMD方法的基于CPU的实现。使用pH副本交换滴定模拟了实验已知的p K a的4种小蛋白和7种酶中的Asp,Glu和His侧链的方法,测试了方法的性能,其中一些与模型值大不相同。对于数据集,由于CpHMD滴定而导致的增加的计算成本在11%到33%的范围内,并且随着可滴定位点与溶质原子数之间的比率大致线性地缩放。将每个副本采样使用2 ns的实验值和计算出的p K a进行比较,得出的平均无符号误差为0.70,均方根误差为0.91,线性相关系数为0.79。尽管此精度水平与CHARMM中基于GBSW的CpHMD相似,但与后者相反,当前的实现方式能够重现p K a的实验顺序。是偶合的羧基二元化合物。我们对采样误差进行了量化,这表明需要长时间的模拟才能使参与盐桥样相互作用或深埋在蛋白质内部的几个可滴定基团的p K a收敛。我们的基准数据表明,GBNeck2-CpHMD是蛋白质p K a预测的有吸引力的工具。







































 京公网安备 11010802027423号
京公网安备 11010802027423号