当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Understanding of Uranyl Ion Complexation on Montmorillonite Edges: A Combined First-Principles Molecular Dynamics–Surface Complexation Modeling Approach
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-16 , DOI: 10.1021/acs.est.8b02504 Chi Zhang 1 , Xiandong Liu 1 , Ruth M. Tinnacher 2, 3 , Christophe Tournassat 2, 3, 4, 5
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-16 , DOI: 10.1021/acs.est.8b02504 Chi Zhang 1 , Xiandong Liu 1 , Ruth M. Tinnacher 2, 3 , Christophe Tournassat 2, 3, 4, 5
Affiliation
|
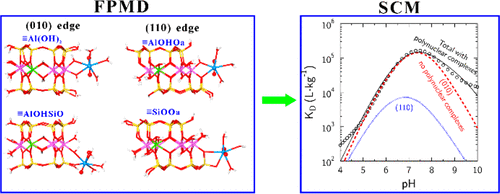
Systematic first-principles molecular dynamics (FPMD) simulations were carried out to study the structures, free energies, and acidity constants of UO22+ surface complexes on montmorillonite in order to elucidate the surface complexation mechanisms of the uranyl ion (UO22+) on clay mineral edges at the atomic scale. Four representative complexing sites were investigated, that is, ≡Al(OH)2 and ≡AlOHSiO on the (010) surface and ≡AlOHOa and ≡SiOOa on the (110) surface. The results show that uranyl ions form bidentate complexes on these sites. All calculated binding free energies for these complexes are very similar. These bidentate complexes can be hydrolyzed, and their corresponding derived pKa values (around 5.0 and 9.0 for pKa1 and pKa2, respectively) indicate that UO2(OH)+ and UO2(OH)2 surface groups are the dominant surface species in the environmental pH range. The OH groups of UO2(OH)2 surface complexes can act as complexing sites for subsequent metals. Additional simulations showed that such multinuclear adsorption is feasible and can be important at high pH. Furthermore, FPMD simulation results served as input parameters for an electrostatic thermodynamic surface complexation model (SCM) that adequately reproduced adsorption data from the literature. Overall, this study provides an improved understanding of UO22+ complexation on clay mineral edge surfaces.
中文翻译:

机械理解的蒙脱石边缘上的铀酰离子络合:结合的第一原理分子动力学-表面络合建模方法
进行系统的第一性原理分子动力学(FPMD)模拟以研究蒙脱石上UO 2 2+表面复合物的结构,自由能和酸度常数,以阐明铀酰离子(UO 2 2+)在原子尺度上的粘土矿物边缘上。研究了四个代表性的络合位点,即(010)表面上的≡Al(OH)2和≡AlOHSiO,以及(110)表面上的andAlOHOa和≡SiOOa。结果表明,铀酰离子在这些位点上形成双齿络合物。这些配合物的所有计算的结合自由能都非常相似。这些二齿配合物可以被水解,其相应的衍生的p K a值(p K a1和p K a2分别约为5.0和9.0 )表明UO 2(OH)+和UO 2(OH)2表面基团是环境pH范围内的主要表面物质。UO 2(OH)2的OH基表面配合物可以充当后续金属的配合位点。附加的模拟表明,这种多核吸附是可行的,并且在高pH值下可能很重要。此外,FPMD模拟结果可作为静电热力学表面络合模型(SCM)的输入参数,该模型可充分再现文献中的吸附数据。总的来说,这项研究提供了对粘土矿物边缘表面上的UO 2 2+络合的更好的理解。
更新日期:2018-07-18
中文翻译:

机械理解的蒙脱石边缘上的铀酰离子络合:结合的第一原理分子动力学-表面络合建模方法
进行系统的第一性原理分子动力学(FPMD)模拟以研究蒙脱石上UO 2 2+表面复合物的结构,自由能和酸度常数,以阐明铀酰离子(UO 2 2+)在原子尺度上的粘土矿物边缘上。研究了四个代表性的络合位点,即(010)表面上的≡Al(OH)2和≡AlOHSiO,以及(110)表面上的andAlOHOa和≡SiOOa。结果表明,铀酰离子在这些位点上形成双齿络合物。这些配合物的所有计算的结合自由能都非常相似。这些二齿配合物可以被水解,其相应的衍生的p K a值(p K a1和p K a2分别约为5.0和9.0 )表明UO 2(OH)+和UO 2(OH)2表面基团是环境pH范围内的主要表面物质。UO 2(OH)2的OH基表面配合物可以充当后续金属的配合位点。附加的模拟表明,这种多核吸附是可行的,并且在高pH值下可能很重要。此外,FPMD模拟结果可作为静电热力学表面络合模型(SCM)的输入参数,该模型可充分再现文献中的吸附数据。总的来说,这项研究提供了对粘土矿物边缘表面上的UO 2 2+络合的更好的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号