当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of Novel Functionally Important Aromatic Residue Interactions in the Extracellular Domain of the Glycine Receptor
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00425 Bijun Tang 1 , Steven O Devenish 1 , Sarah C R Lummis 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00425 Bijun Tang 1 , Steven O Devenish 1 , Sarah C R Lummis 1
Affiliation
|
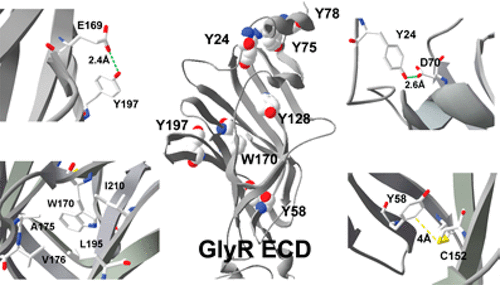
The extracellular domains (ECDs) of Cys-loop receptors contain many aromatic amino acids, but only relatively few have been well studied. Here we explore the roles of Tyr and Trp residues in the ECD of the glycine receptor and show that four such residues that have not been previously studied (Y24, Y58, W170, and Y197) contribute significantly to the function of the protein. The residues were studied by creating mutant receptors, characterizing them using two-electrode voltage clamp in Xenopus oocytes, and interpreting changes in receptor parameters using structural information about the open and closed states of the receptor. Alanine substitution of all these residues ablates function or increases the glycine EC50. There are also a number of changes in the relative maximal responses to taurine, a partial agonist, compared to glycine. Further mutations, in combination with structural information, suggest Y24 contributes to an anion−π interaction with a binding loop D residue, Y58 to an S−π interaction stabilizing the Cys loop, W170 to hydrophobic interactions stabilizing the hydrophobic interior of the subunit, and Y197 to a hydrogen bond linking binding loops B and C. These interactions appear to be broadly conserved in other Cys-loop receptors. Thus, we have identified new regions of the glycine receptor that are important contributors to receptor activation and are likely also to contribute to function in other members of this important protein family.
中文翻译:

甘氨酸受体细胞外结构域中新的功能重要的芳香残基相互作用的鉴定
Cys 环受体的细胞外结构域 (ECD) 含有许多芳香族氨基酸,但只有相对较少的部分得到了很好的研究。在这里,我们探讨了 Tyr 和 Trp 残基在甘氨酸受体的 ECD 中的作用,并表明以前没有研究过的四个这样的残基(Y24、Y58、W170 和 Y197)对蛋白质的功能有显着贡献。通过创建突变受体来研究残基,使用非洲爪蟾卵母细胞中的双电极电压钳对其进行表征,并使用有关受体打开和关闭状态的结构信息解释受体参数的变化。所有这些残基的丙氨酸取代消除功能或增加甘氨酸 EC 50. 与甘氨酸相比,对牛磺酸(一种部分激动剂)的相对最大反应也有许多变化。进一步的突变,结合结构信息,表明 Y24 有助于与结合环 D 残基的阴离子-π 相互作用,Y58 有助于稳定 Cys 环的 S-π 相互作用,W170 有助于稳定亚基疏水内部的疏水相互作用,以及Y197 与连接环 B 和 C 的氢键连接。这些相互作用似乎在其他 Cys 环受体中广泛保守。因此,我们已经确定了甘氨酸受体的新区域,这些区域是受体激活的重要贡献者,也可能有助于这一重要蛋白质家族的其他成员的功能。
更新日期:2018-06-27
中文翻译:

甘氨酸受体细胞外结构域中新的功能重要的芳香残基相互作用的鉴定
Cys 环受体的细胞外结构域 (ECD) 含有许多芳香族氨基酸,但只有相对较少的部分得到了很好的研究。在这里,我们探讨了 Tyr 和 Trp 残基在甘氨酸受体的 ECD 中的作用,并表明以前没有研究过的四个这样的残基(Y24、Y58、W170 和 Y197)对蛋白质的功能有显着贡献。通过创建突变受体来研究残基,使用非洲爪蟾卵母细胞中的双电极电压钳对其进行表征,并使用有关受体打开和关闭状态的结构信息解释受体参数的变化。所有这些残基的丙氨酸取代消除功能或增加甘氨酸 EC 50. 与甘氨酸相比,对牛磺酸(一种部分激动剂)的相对最大反应也有许多变化。进一步的突变,结合结构信息,表明 Y24 有助于与结合环 D 残基的阴离子-π 相互作用,Y58 有助于稳定 Cys 环的 S-π 相互作用,W170 有助于稳定亚基疏水内部的疏水相互作用,以及Y197 与连接环 B 和 C 的氢键连接。这些相互作用似乎在其他 Cys 环受体中广泛保守。因此,我们已经确定了甘氨酸受体的新区域,这些区域是受体激活的重要贡献者,也可能有助于这一重要蛋白质家族的其他成员的功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号