当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Peroxidase versus Peroxygenase Activity: Substrate Substituent Effects as Modulators of Enzyme Function in the Multifunctional Catalytic Globin Dehaloperoxidase
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00540 Ashlyn H. McGuire 1 , Leiah M. Carey 1 , Vesna de Serrano 1 , Safaa Dali 1 , Reza A. Ghiladi 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00540 Ashlyn H. McGuire 1 , Leiah M. Carey 1 , Vesna de Serrano 1 , Safaa Dali 1 , Reza A. Ghiladi 1
Affiliation
|
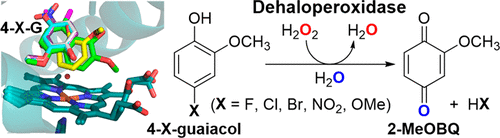
The dehaloperoxidase-hemoglobin (DHP) from the terebellid polychaete Amphitrite ornata is a multifunctional hemoprotein that catalyzes the oxidation of a wide variety of substrates, including halo/nitrophenols, haloindoles, and pyrroles, via peroxidase and/or peroxygenase mechanisms. To probe whether substrate substituent effects can modulate enzyme activity in DHP, we investigated its reactiviy against a panel of o-guaiacol substrates given their presence (from native/halogenated and non-native/anthropogenic sources) in the benthic environment that A. ornata inhabits. Using biochemical assays supported by spectroscopic, spectrometric, and structural studies, DHP was found to catalyze the H2O2-dependent oxidative dehalogenation of 4-haloguaiacols (F, Cl, and Br) to 2-methoxybenzoquinone (2-MeOBQ). 18O labeling studies confirmed that O atom incorporation was derived exclusively from water, consistent with substrate oxidation via a peroxidase-based mechanism. The 2-MeOBQ product further reduced DHP to its oxyferrous state, providing a link between the substrate oxidation and O2 carrier functions of DHP. Nonnative substrates resulted in polymerization of the initial substrate with varying degrees of oxidation, with 2-MeOBQ identified as a minor product. When viewed alongside the reactivity of previously studied phenolic substrates, the results presented here show that simple substituent effects can serve as functional switches between peroxidase and peroxygenase activities in this multifunctional catalytic globin. More broadly, when recent findings on DHP activity with nitrophenols and azoles are included, the results presented here further demonstrate the breadth of heterocyclic compounds of anthropogenic origin that can potentially disrupt marine hemoglobins or function as environmental stressors, findings that may be important when assessing the environmental impact of these pollutants (and their metabolites) on aquatic systems.
中文翻译:

过氧化物酶与过氧化酶活性:底物替代作用作为多功能催化球蛋白脱卤过氧化物酶中酶功能的调节剂。
得自于萜类多毛小动物的Amphitrite ornata的脱卤过氧化物酶-血红蛋白(DHP)是一种多功能的血蛋白,可通过过氧化物酶和/或过氧合酶机制催化多种底物的氧化,包括卤代/硝基酚,卤代吲哚和吡咯。以探测衬底取代基效应是否可以调节DHP酶活性,我们研究了其对reactiviy的面板直径:给定它们的存在-guaiacol底物(由天然/卤化和非天然的/人为来源)在海底环境A.姬蛙栖息。使用光谱学,光谱学和结构研究支持的生化分析,发现DHP催化H 2 O 2依赖性的4-卤愈创木酚(F,Cl和Br)氧化脱卤为2-甲氧基苯醌(2-MeOBQ)。18 O标记研究证实,O原子的掺入仅源自水,这与基于过氧化物酶的底物氧化相一致。2-MeOBQ产物进一步将DHP还原为其氧化亚铁态,提供了底物氧化与O 2之间的联系DHP的载体功能。非天然底物导致初始底物发生不同程度的氧化聚合,其中2-MeOBQ被鉴定为次要产物。当与先前研究的酚类底物的反应性一起观察时,此处显示的结果表明,简单的取代基作用可以充当该多功能催化球蛋白中过氧化物酶和过氧化酶活性之间的功能转换。更广泛地讲,当包括关于硝基苯酚和吡咯的DHP活性的最新发现时,此处显示的结果进一步证明了人为来源的杂环化合物的广度,该化合物可能破坏海洋血红蛋白或充当环境应激源,
更新日期:2018-06-27
中文翻译:

过氧化物酶与过氧化酶活性:底物替代作用作为多功能催化球蛋白脱卤过氧化物酶中酶功能的调节剂。
得自于萜类多毛小动物的Amphitrite ornata的脱卤过氧化物酶-血红蛋白(DHP)是一种多功能的血蛋白,可通过过氧化物酶和/或过氧合酶机制催化多种底物的氧化,包括卤代/硝基酚,卤代吲哚和吡咯。以探测衬底取代基效应是否可以调节DHP酶活性,我们研究了其对reactiviy的面板直径:给定它们的存在-guaiacol底物(由天然/卤化和非天然的/人为来源)在海底环境A.姬蛙栖息。使用光谱学,光谱学和结构研究支持的生化分析,发现DHP催化H 2 O 2依赖性的4-卤愈创木酚(F,Cl和Br)氧化脱卤为2-甲氧基苯醌(2-MeOBQ)。18 O标记研究证实,O原子的掺入仅源自水,这与基于过氧化物酶的底物氧化相一致。2-MeOBQ产物进一步将DHP还原为其氧化亚铁态,提供了底物氧化与O 2之间的联系DHP的载体功能。非天然底物导致初始底物发生不同程度的氧化聚合,其中2-MeOBQ被鉴定为次要产物。当与先前研究的酚类底物的反应性一起观察时,此处显示的结果表明,简单的取代基作用可以充当该多功能催化球蛋白中过氧化物酶和过氧化酶活性之间的功能转换。更广泛地讲,当包括关于硝基苯酚和吡咯的DHP活性的最新发现时,此处显示的结果进一步证明了人为来源的杂环化合物的广度,该化合物可能破坏海洋血红蛋白或充当环境应激源,



























 京公网安备 11010802027423号
京公网安备 11010802027423号