当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Top-Down Characterization of Proteins with Intact Disulfide Bonds Using Activated-Ion Electron Transfer Dissociation
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.analchem.8b01113 Matthew J P Rush 1, 2 , Nicholas M Riley 1, 2 , Michael S Westphall 1 , Joshua J Coon 1, 2, 3, 4
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-06-27 00:00:00 , DOI: 10.1021/acs.analchem.8b01113 Matthew J P Rush 1, 2 , Nicholas M Riley 1, 2 , Michael S Westphall 1 , Joshua J Coon 1, 2, 3, 4
Affiliation
|
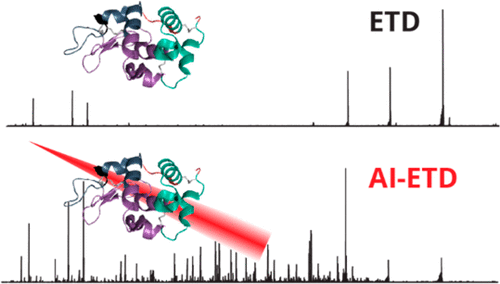
Here we report the fragmentation of disulfide linked intact proteins using activated-ion electron transfer dissociation (AI-ETD) for top-down protein characterization. This fragmentation method is then compared to the alternative methods of beam-type collisional activation (HCD), electron transfer dissociation (ETD), and electron transfer and higher-energy collision dissociation (EThcD). We analyzed multiple precursor charge states of the protein standards bovine insulin, α-lactalbumin, lysozyme, β-lactoglobulin, and trypsin inhibitor. In all cases, we found that AI-ETD provides a boost in protein sequence coverage information and the generation of fragment ions from within regions enclosed by disulfide bonds. AI-ETD shows the largest improvement over the other techniques when analyzing highly disulfide linked and low charge density precursor ions. This substantial improvement is attributed to the concurrent irradiation of the gas phase ions while the electron-transfer reaction is taking place, mitigating nondissociative electron transfer, helping unfold the gas phase protein during the electron transfer event, and preventing disulfide bond reformation. We also show that AI-ETD is able to yield comparable sequence coverage information when disulfide bonds are left intact relative to proteins that have been reduced and alkylated. This work demonstrates that AI-ETD is an effective fragmentation method for the analysis of proteins with intact disulfide bonds, dramatically enhancing sequence ion generation and total sequence coverage compared to HCD and ETD.
中文翻译:

使用活化离子电子转移解离对具有完整二硫键的蛋白质进行自上而下的表征
在这里,我们报告使用活化离子电子转移解离 (AI-ETD) 对二硫键连接的完整蛋白质进行碎片化,以进行自上而下的蛋白质表征。然后将该碎裂方法与束型碰撞激活 (HCD)、电子转移解离 (ETD) 以及电子转移和高能碰撞解离 (EThcD) 等替代方法进行比较。我们分析了蛋白质标准品牛胰岛素、α-乳清蛋白、溶菌酶、β-乳球蛋白和胰蛋白酶抑制剂的多种前体电荷态。在所有情况下,我们发现 AI-ETD 增强了蛋白质序列覆盖信息,并从二硫键包围的区域内生成碎片离子。在分析高度二硫键连接和低电荷密度前体离子时,AI-ETD 比其他技术显示出最大的改进。这种实质性的改进归因于电子转移反应发生时气相离子的同时照射,减轻了非解离电子转移,有助于在电子转移事件期间展开气相蛋白质,并防止二硫键重组。我们还表明,当二硫键相对于已还原和烷基化的蛋白质保持完整时,AI-ETD 能够产生可比的序列覆盖信息。这项工作表明,AI-ETD 是一种用于分析具有完整二硫键的蛋白质的有效裂解方法,与 HCD 和 ETD 相比,显着增强了序列离子生成和总序列覆盖率。
更新日期:2018-06-27
中文翻译:

使用活化离子电子转移解离对具有完整二硫键的蛋白质进行自上而下的表征
在这里,我们报告使用活化离子电子转移解离 (AI-ETD) 对二硫键连接的完整蛋白质进行碎片化,以进行自上而下的蛋白质表征。然后将该碎裂方法与束型碰撞激活 (HCD)、电子转移解离 (ETD) 以及电子转移和高能碰撞解离 (EThcD) 等替代方法进行比较。我们分析了蛋白质标准品牛胰岛素、α-乳清蛋白、溶菌酶、β-乳球蛋白和胰蛋白酶抑制剂的多种前体电荷态。在所有情况下,我们发现 AI-ETD 增强了蛋白质序列覆盖信息,并从二硫键包围的区域内生成碎片离子。在分析高度二硫键连接和低电荷密度前体离子时,AI-ETD 比其他技术显示出最大的改进。这种实质性的改进归因于电子转移反应发生时气相离子的同时照射,减轻了非解离电子转移,有助于在电子转移事件期间展开气相蛋白质,并防止二硫键重组。我们还表明,当二硫键相对于已还原和烷基化的蛋白质保持完整时,AI-ETD 能够产生可比的序列覆盖信息。这项工作表明,AI-ETD 是一种用于分析具有完整二硫键的蛋白质的有效裂解方法,与 HCD 和 ETD 相比,显着增强了序列离子生成和总序列覆盖率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号