当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High Thermal Stability of Oligomeric Assemblies of Thermophilic Rhodopsin in a Lipid Environment
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-27 , DOI: 10.1021/acs.jpcb.8b04894 Tomomi Shionoya 1 , Misao Mizuno 1 , Takashi Tsukamoto 2 , Kento Ikeda , Hayato Seki 3 , Keiichi Kojima 2 , Mikihiro Shibata , Izuru Kawamura 3 , Yuki Sudo 2 , Yasuhisa Mizutani 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-27 , DOI: 10.1021/acs.jpcb.8b04894 Tomomi Shionoya 1 , Misao Mizuno 1 , Takashi Tsukamoto 2 , Kento Ikeda , Hayato Seki 3 , Keiichi Kojima 2 , Mikihiro Shibata , Izuru Kawamura 3 , Yuki Sudo 2 , Yasuhisa Mizutani 1
Affiliation
|
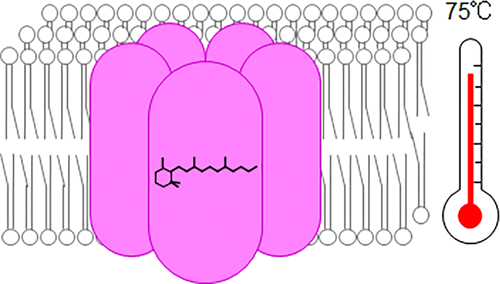
Thermophilic rhodopsin (TR) is a light-driven proton pump from the extreme thermophile Thermus thermophilus JL-18. Previous studies on TR solubilized with detergent showed that the protein exhibits high thermal stability and forms a trimer at room temperature but irreversibly dissociates into monomers when incubated at physiological temperature (75 °C). In the present study, we used resonance Raman (RR) spectroscopy, solid-state NMR spectroscopy, and high-speed atomic force microscopy to analyze the oligomeric structure of TR in a lipid environment. The obtained spectra and microscopic images demonstrate that TR adopts a pentameric form in a lipid environment and that this assembly is stable at the physiological temperature, in contrast to the behavior of the protein in the solubilized state. These results indicate that the thermal stability of the oligomeric assembly of TR is higher in a lipid environment than in detergent micelles. The observed RR spectra also showed that the retinal chromophore is strongly hydrogen bonded to an internal water molecule via a protonated Schiff base, which is characteristic of proton-pumping rhodopsins. The obtained data strongly suggest that TR functions in the pentameric form at physiological temperature in the extreme thermophile T. thermophilus JL-18. We utilized the high thermal stability of the monomeric form of solubilized TR and here report the first RR spectra of the monomeric form of a microbial rhodopsin. The observed RR spectra revealed that the monomerization of TR alters the chromophore structure: there are changes in the bond alternation of the polyene chain and in the hydrogen-bond strength of the protonated Schiff base. The present study revealed the high thermal stability of oligomeric assemblies of TR in the lipid environment and suggested the importance of using TR embedded in lipid membrane for elucidation of its functional mechanism.
中文翻译:

脂质环境中嗜热视紫红质的寡聚体组件的高热稳定性
嗜热视紫红质(TR)是来自极端嗜热菌Thermus thermophilus的光驱动质子泵JL-18。以前用去污剂溶解的TR的研究表明,该蛋白在室温下显示出高的热稳定性并形成三聚体,但在生理温度(75°C)下孵育时不可逆地分解成单体。在本研究中,我们使用共振拉曼(RR)光谱,固态NMR光谱和高速原子力显微镜来分析脂质环境中TR的低聚物结构。所获得的光谱和显微图像表明,TR在脂质环境中采用五聚体形式,并且该组装在生理温度下是稳定的,这与蛋白质在增溶状态下的行为相反。这些结果表明,在脂质环境中,TR的寡聚体组装体的热稳定性高于去污剂胶束中的热稳定性。观察到的RR光谱还表明,视网膜发色团通过质子化的席夫碱牢固地与内部水分子氢键结合,这是质子泵浦视紫红质的特征。获得的数据强烈表明TR在极端嗜热菌的生理温度下以五聚体形式起作用。嗜热链球菌JL-18。我们利用了溶解态TR的单体形式的高热稳定性,并在此报告了微生物视紫红质的单体形式的第一个RR光谱。观察到的RR光谱表明TR的单体化改变了发色团的结构:多烯链的键交替和质子化席夫碱的氢键强度都有变化。本研究揭示了脂类环境中TR的寡聚体组件具有很高的热稳定性,并提出了使用嵌入脂质膜中的TR阐明其功能机制的重要性。
更新日期:2018-06-28
中文翻译:

脂质环境中嗜热视紫红质的寡聚体组件的高热稳定性
嗜热视紫红质(TR)是来自极端嗜热菌Thermus thermophilus的光驱动质子泵JL-18。以前用去污剂溶解的TR的研究表明,该蛋白在室温下显示出高的热稳定性并形成三聚体,但在生理温度(75°C)下孵育时不可逆地分解成单体。在本研究中,我们使用共振拉曼(RR)光谱,固态NMR光谱和高速原子力显微镜来分析脂质环境中TR的低聚物结构。所获得的光谱和显微图像表明,TR在脂质环境中采用五聚体形式,并且该组装在生理温度下是稳定的,这与蛋白质在增溶状态下的行为相反。这些结果表明,在脂质环境中,TR的寡聚体组装体的热稳定性高于去污剂胶束中的热稳定性。观察到的RR光谱还表明,视网膜发色团通过质子化的席夫碱牢固地与内部水分子氢键结合,这是质子泵浦视紫红质的特征。获得的数据强烈表明TR在极端嗜热菌的生理温度下以五聚体形式起作用。嗜热链球菌JL-18。我们利用了溶解态TR的单体形式的高热稳定性,并在此报告了微生物视紫红质的单体形式的第一个RR光谱。观察到的RR光谱表明TR的单体化改变了发色团的结构:多烯链的键交替和质子化席夫碱的氢键强度都有变化。本研究揭示了脂类环境中TR的寡聚体组件具有很高的热稳定性,并提出了使用嵌入脂质膜中的TR阐明其功能机制的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号