当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox Active Quinoidal 1,2,4-Benzotriazines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.joc.8b01311 Georgia A. Zissimou 1 , Andreas Kourtellaris 1 , Maria Manoli 1 , Panayiotis A. Koutentis 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.joc.8b01311 Georgia A. Zissimou 1 , Andreas Kourtellaris 1 , Maria Manoli 1 , Panayiotis A. Koutentis 1
Affiliation
|
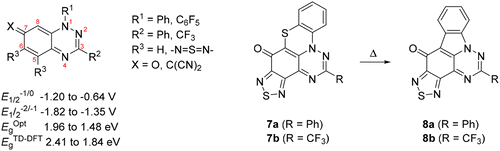
Modifying the para-quinonimine 1,3-diphenyl-1,2,4-benzotriazin-7(1H)-one (2a) (E1/2–1/0 −1.20 V), by replacing the N1-phenyl by pentafluorophenyl, the C3-phenyl by trifluoromethyl, or the C7 carbonyl by ylidenemalononitrile, led to improved electron affinities as determined by cyclic voltammetry and computational studies. Combining structural changes further improved electron accepting abilities: the most electron deficient analogues (E1/2–1/0 ∼ −0.65 V) involved combining the ylidenemalononitrile groups at C7 with the trifluoromethyl groups at C3. 1,2,5-Thiadiazolo fusion at C5–C6 did not affect the redox behavior but did enhance the UV–vis absorption profile. During the synthesis of the thiadiazolo analogues, 1,4-thiazino-fused analogues 6 were obtained in low yield, which thermally ring contract to the triazafluoranthenones 7. Compounds are fully characterized, and X-ray data are provided for selected analogues.
中文翻译:

氧化还原活性喹啉1,2,4-苯并三嗪
通过将N1-苯基替换为N1-苯基,修饰对-喹啉亚胺1,3-二苯基-1,2,4-苯并三嗪-7(1 H)-一(2a)(E 1/2 –1/0 -1.20 V)通过循环伏安法和计算研究确定,五氟苯基,三氟甲基的C3-苯基或亚甲基丙二腈的C7羰基可改善电子亲和力。结合结构变化进一步提高了电子接受能力:最缺乏电子的类似物(E 1/2 –1/0约-0.65V)涉及将C7处的亚甲基丙二腈基团与C3处的三氟甲基基团结合。在C5–C6处的1,2,5-噻二唑融合不会影响氧化还原行为,但会增强UV-vis吸收特性。在噻二唑类似物的合成过程中,以低产率获得了1,4-噻嗪基融合的类似物6,其热环收缩至三氮杂蒽酮7。对化合物进行了充分表征,并提供了所选类似物的X射线数据。
更新日期:2018-06-25
中文翻译:

氧化还原活性喹啉1,2,4-苯并三嗪
通过将N1-苯基替换为N1-苯基,修饰对-喹啉亚胺1,3-二苯基-1,2,4-苯并三嗪-7(1 H)-一(2a)(E 1/2 –1/0 -1.20 V)通过循环伏安法和计算研究确定,五氟苯基,三氟甲基的C3-苯基或亚甲基丙二腈的C7羰基可改善电子亲和力。结合结构变化进一步提高了电子接受能力:最缺乏电子的类似物(E 1/2 –1/0约-0.65V)涉及将C7处的亚甲基丙二腈基团与C3处的三氟甲基基团结合。在C5–C6处的1,2,5-噻二唑融合不会影响氧化还原行为,但会增强UV-vis吸收特性。在噻二唑类似物的合成过程中,以低产率获得了1,4-噻嗪基融合的类似物6,其热环收缩至三氮杂蒽酮7。对化合物进行了充分表征,并提供了所选类似物的X射线数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号